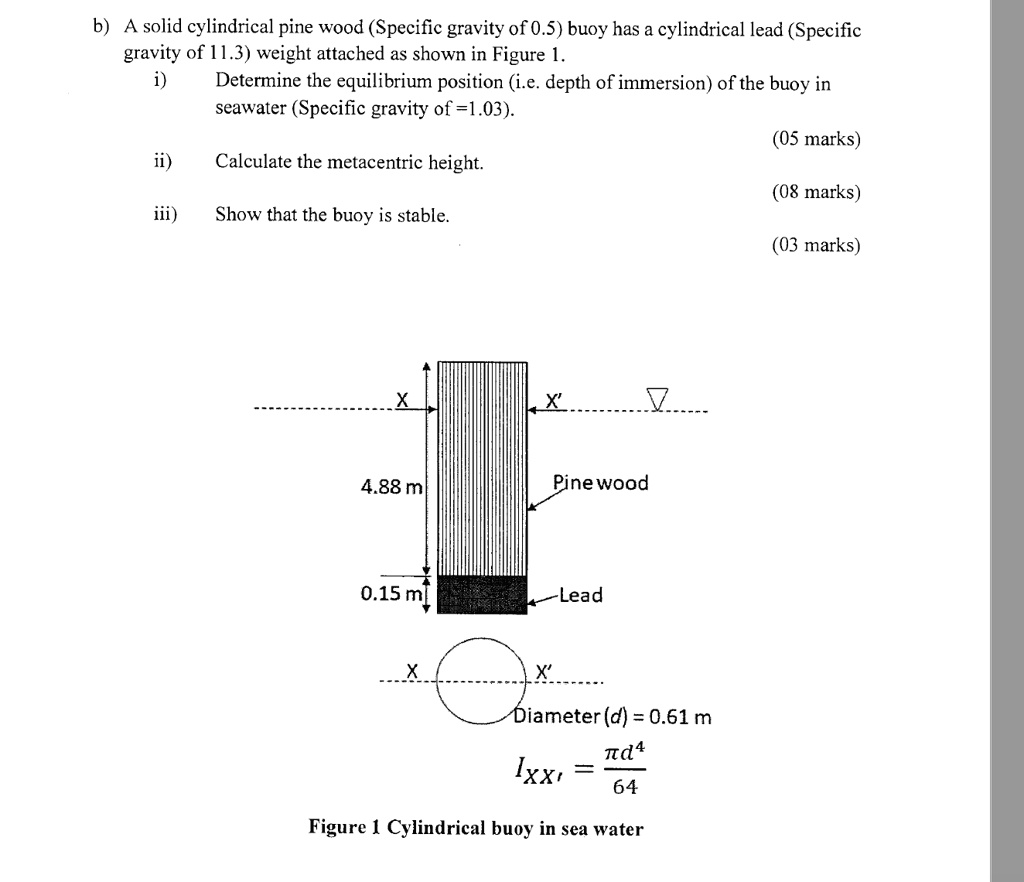
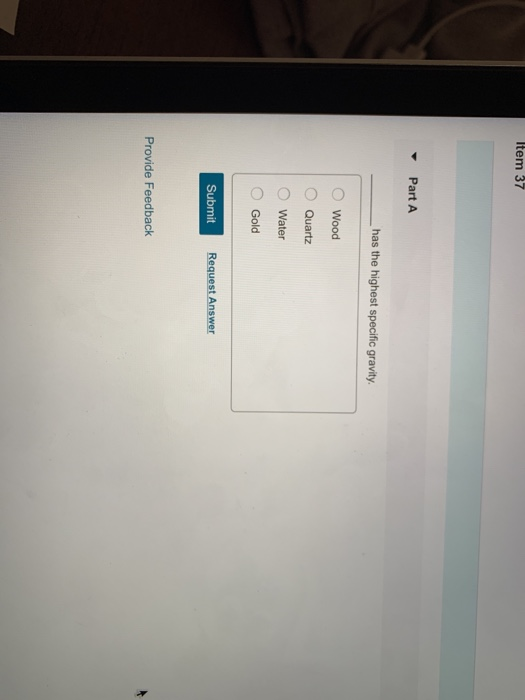
Which Of The Following Has The Highest Specific Gravity

The question of which common substance possesses the highest specific gravity often sparks curiosity and debate, moving beyond simple science trivia into practical applications across industries.
This article delves into the realm of density, comparing various substances to determine which emerges as the densest, clarifying common misconceptions along the way.
Understanding Specific Gravity
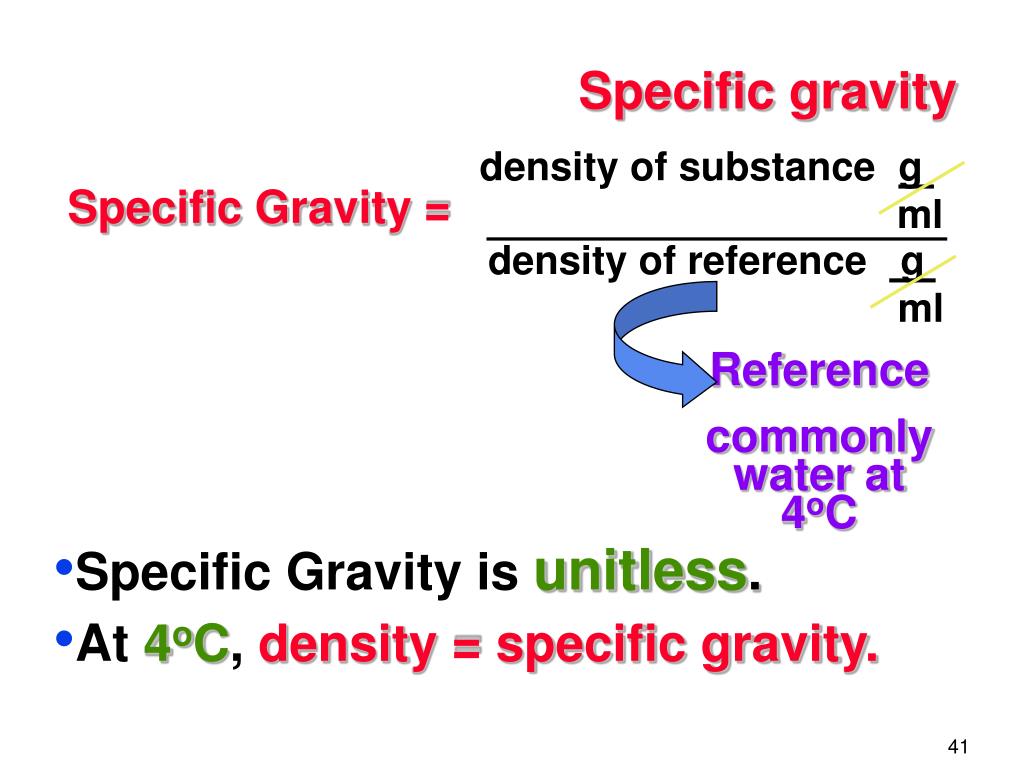
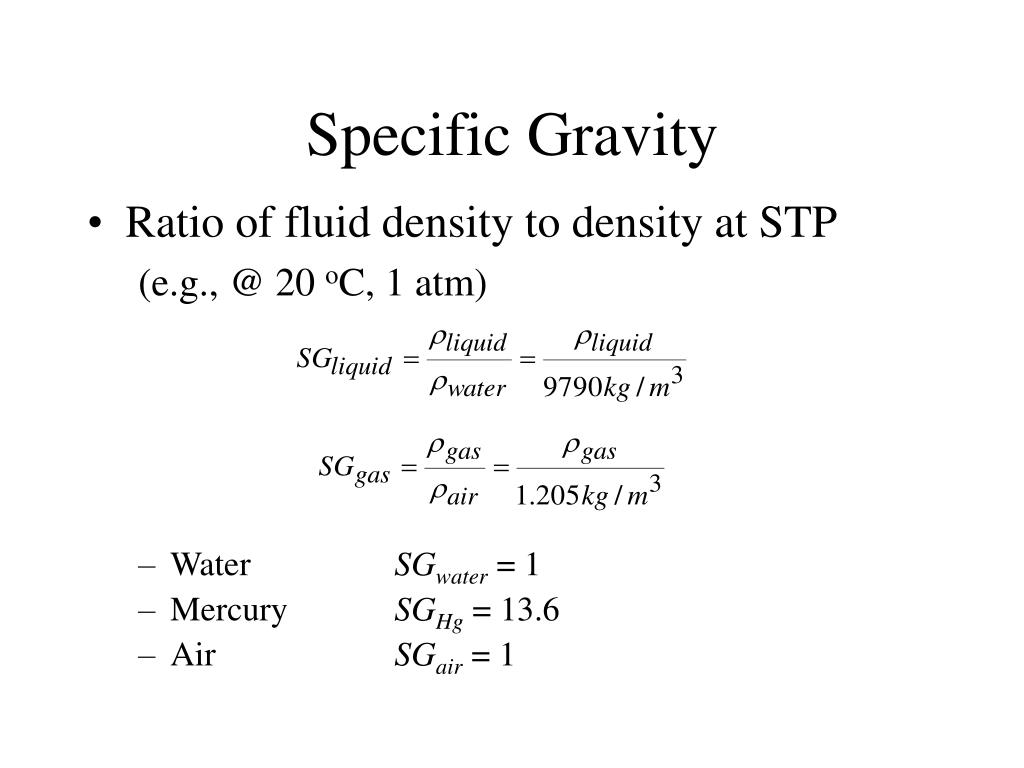
Specific gravity, also known as relative density, is the ratio of the density of a substance to the density of a reference substance, typically water for liquids. It is a dimensionless quantity, meaning it has no units. A specific gravity greater than 1 indicates that the substance is denser than water, while a specific gravity less than 1 indicates it is less dense.
Understanding specific gravity is crucial in various fields, including geology, chemistry, and engineering. It is used in identifying minerals, assessing the purity of substances, and designing structures that can withstand specific weights and pressures.
The Contenders: Common Substances and Their Specific Gravities
Several common substances are often compared when discussing specific gravity. These include gold, lead, iron, aluminum, and even everyday liquids like mercury and water. Each has a distinct density and therefore, a different specific gravity.
Water, the reference point, has a specific gravity of 1. This means that a substance with a specific gravity of 2 is twice as dense as water.
Aluminum, a lightweight metal widely used in construction and manufacturing, has a specific gravity of approximately 2.7. Iron, another common metal, possesses a specific gravity of around 7.87.
Lead, known for its use in batteries and radiation shielding, boasts a significantly higher specific gravity, approximately 11.34. However, even lead is not the densest among commonly known substances.
Gold, prized for its beauty and resistance to corrosion, has a specific gravity of around 19.3. This makes it considerably denser than lead, iron, or aluminum.
The Winner: Osmium
While gold is remarkably dense, it is not the champion in this contest. The title of highest specific gravity among commonly referenced substances belongs to osmium.
Osmium, a hard, brittle, bluish-white transition metal in the platinum group, boasts a specific gravity of approximately 22.59. This makes it significantly denser than gold, placing it at the top of the list.
Iridium, another platinum group metal, is nearly as dense as osmium, with a specific gravity around 22.65 (slightly higher depending on allotropic form and measurement conditions).
However, osmium is more frequently cited in comparisons of specific gravity due to its higher mass density in its most commonly available form.
Why This Matters
Understanding specific gravity is not merely an academic exercise. It has real-world implications in various fields.
Geologists use specific gravity to identify minerals, helping them understand the composition of the Earth's crust. Engineers rely on this property when designing structures, ensuring they can withstand the stresses imposed by different materials.
Furthermore, specific gravity is used in the quality control of manufactured goods. Variations in density can indicate defects or inconsistencies in the production process.
Conclusion
While many substances possess interesting densities, osmium stands out with the highest specific gravity among those commonly discussed.
Its exceptional density is a testament to the unique properties of this element and its place in the periodic table. Though iridium is comparably dense under specific conditions, osmium maintains its reputation for exceptionally high density.
The study of specific gravity continues to be a vital aspect of scientific inquiry, driving innovation and discovery across various disciplines.






.PNG)
.jpg)