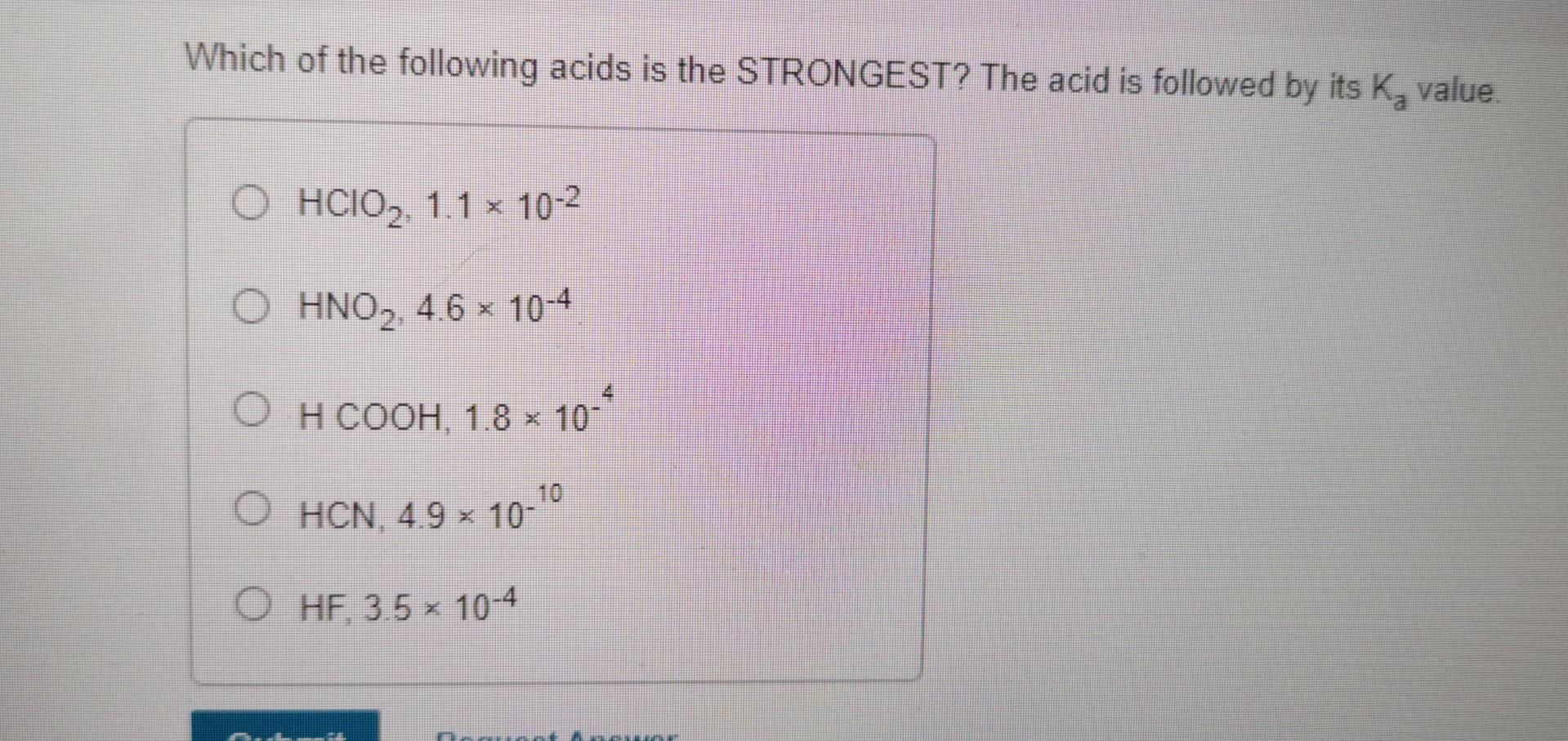
Which Of The Following Is A Strongest Acid
The question of "which acid reigns supreme" isn't merely a theoretical exercise confined to sterile laboratories. It impacts fields ranging from industrial chemistry and materials science to environmental remediation and even pharmaceutical development.
Accurately determining acidity strength is crucial for predicting reaction outcomes, designing effective catalysts, and understanding the corrosive potential of different substances.
The answer to "which is the strongest acid?" is more complex than it initially appears.
Understanding Acidity and pKa Values
At its core, acidity refers to a substance's ability to donate protons (H+) or accept electrons. The more readily a substance donates a proton, the stronger it is considered as an acid.
This ability is quantified using the pKa value, a logarithmic scale where lower values indicate stronger acidity. For instance, an acid with a pKa of -10 is significantly stronger than one with a pKa of 2.
It's important to remember that pKa values are context-dependent, influenced by the solvent and temperature of the solution.
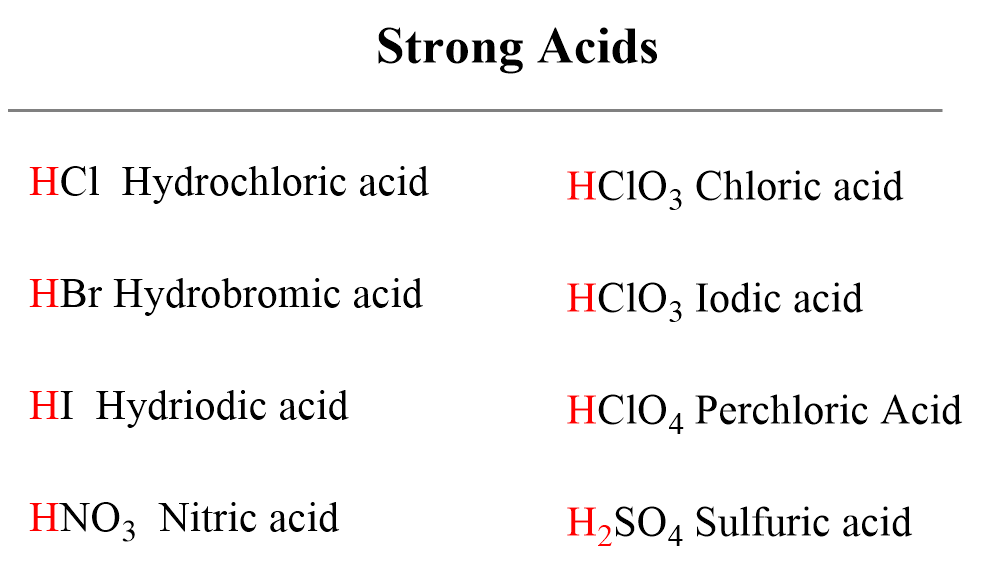
The Usual Suspects: Mineral Acids
Common strong acids often encountered in introductory chemistry include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3).
These mineral acids are powerful proton donors and are extensively used in various industrial processes.
Hydrochloric acid, for example, is vital in steel pickling and pH regulation, while sulfuric acid serves as a key reagent in fertilizer production and chemical synthesis.
Beyond the Basics: Superacids
The realm of acidity extends far beyond these common strong acids into the domain of superacids. These acids are stronger than 100% sulfuric acid (pKa ~ -3).
Superacids owe their extreme acidity to specific molecular structures and synergistic combinations of different acidic components.
One of the most well-known superacids is fluoroantimonic acid (HSbF6), a mixture of hydrogen fluoride (HF) and antimony pentafluoride (SbF5).
Fluoroantimonic Acid: A Prime Contender
Fluoroantimonic acid is an exceptionally potent acid, with estimates placing its acidity at up to 1016 times stronger than 100% sulfuric acid.
The extraordinary strength stems from the synergistic interaction between HF and SbF5. SbF5 strongly binds fluoride ions from HF, generating highly reactive and proton-donating species.
This combination creates an environment where protons are exceptionally free to react with other molecules.
According to research published in the Journal of the American Chemical Society, the acidity of fluoroantimonic acid is related to the highly electrophilic nature of the SbF5 component.
Carborane Acids: A Different Approach to Acidity
While fluoroantimonic acid represents one extreme of acidity, another class of superacids, the carborane acids, offer a different approach. These acids are notable for their extremely stable conjugate bases.
Developed by researchers like Christopher Reed, carborane acids consist of boron-cluster anions stabilized by organic substituents.
Their acidity stems from the exceptional delocalization of negative charge in the carborane anion, making it a very weak base and thus rendering the corresponding acid exceptionally strong.
Comparing Strengths: Fluoroantimonic vs. Carborane
The question of whether fluoroantimonic acid or carborane acids are stronger depends on the specific conditions and the definition of "strength" being used.
Fluoroantimonic acid, in its pure form, can be estimated to have a higher Hammett acidity function (a measure of acidity in highly concentrated solutions) than carborane acids.
However, carborane acids have the advantage of forming very weakly coordinating anions, making them useful in stabilizing highly reactive cations.
Applications and Implications
The discovery and characterization of superacids have revolutionized various fields. They are utilized as catalysts in organic synthesis, allowing for reactions previously impossible or requiring harsh conditions.
Superacids also play a crucial role in the petrochemical industry, facilitating the isomerization and alkylation of hydrocarbons.
Furthermore, their study has provided insights into the fundamental nature of acidity and the behavior of molecules in extreme environments.
Safety Considerations
Working with superacids requires extreme caution due to their highly corrosive nature. They can cause severe burns on contact with skin and can react violently with water and other substances.
Specialized equipment, including appropriate personal protective equipment (PPE) and fume hoods, is essential when handling these compounds.
Proper disposal methods must also be followed to minimize environmental risks.
The Future of Acid Research
The search for even stronger acids continues, driven by the potential for new catalytic reactions and materials.
Researchers are exploring novel molecular designs and combinations of acidic components to push the boundaries of acidity.
Understanding the fundamental principles governing acidity will lead to the development of more selective and efficient catalysts for a wide range of applications.
Ultimately, the question of the "strongest acid" is not a static one, but rather an ongoing exploration at the forefront of chemistry.
This exploration promises not only to refine our understanding of chemical reactivity but also to unlock new possibilities in fields ranging from energy production to medicine.






![Which Of The Following Is A Strongest Acid [ANSWERED] Of the following which is the strongest acid Select one O a](https://media.kunduz.com/media/sug-question-candidate/20220505143611627416-4564821.jpg?h=512)