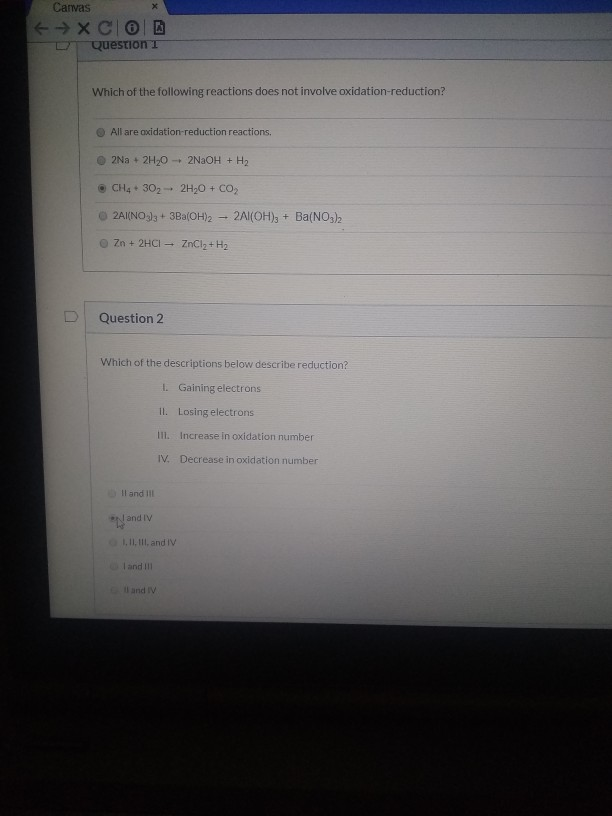
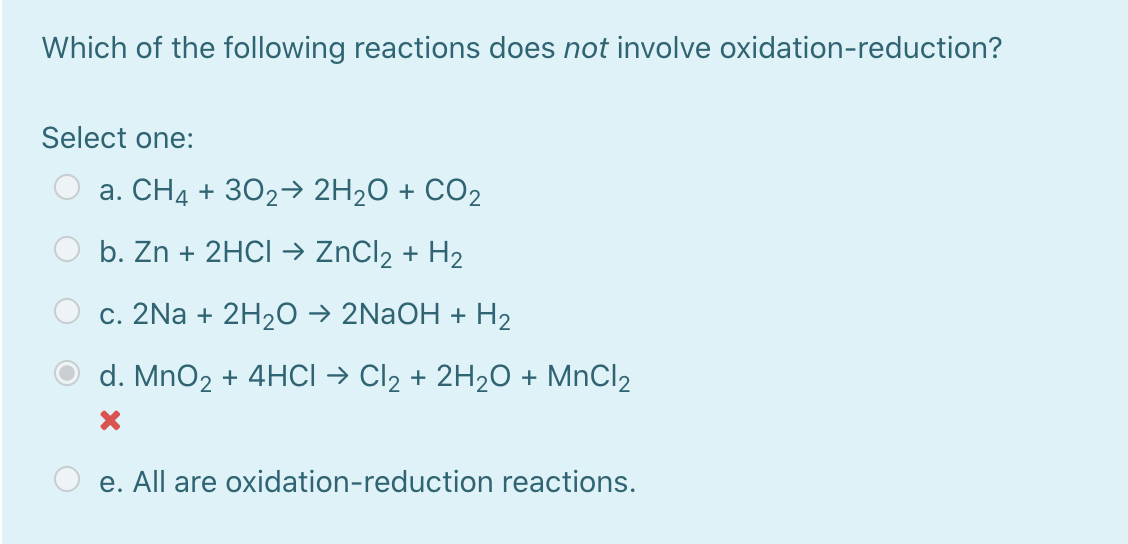
Which Of The Following Reactions Does Not Involve Oxidation Reduction

Imagine a bustling chemistry lab, beakers bubbling, the air thick with the scent of intriguing compounds. A group of students huddles around a whiteboard, their faces illuminated by equations scribbled in colorful markers. The question that has them captivated: “Which of the following reactions does not involve oxidation-reduction?” A deceptively simple question that unravels a complex world of electron transfer and chemical transformations.
This seemingly academic question is actually a gateway to understanding the fundamental processes that drive the world around us. From the rusting of iron to the energy production within our cells, redox reactions are essential for life as we know it. Identifying which reactions *don't* involve these electron exchanges sheds light on alternative chemical pathways and principles.
The Dance of Electrons: Understanding Redox Reactions
Oxidation-reduction, or redox, reactions are at the heart of many chemical processes. They involve the transfer of electrons between chemical species. One species loses electrons (oxidation), while another gains electrons (reduction).
The terms "oxidation" and "reduction" might sound intimidating, but they describe straightforward concepts. Oxidation, originally defined as the reaction of a substance with oxygen, now refers to the loss of electrons. Reduction, originally defined as the removal of oxygen from a substance, now refers to the gain of electrons.
A helpful mnemonic is "OIL RIG": Oxidation Is Loss (of electrons), Reduction Is Gain (of electrons). Remember this, and you're halfway to mastering redox reactions.
Identifying Redox Reactions
How do you spot a redox reaction? Look for changes in oxidation states. The oxidation state, sometimes called the oxidation number, represents the hypothetical charge an atom would have if all bonds were completely ionic.
If an atom's oxidation state increases during a reaction, it's been oxidized. If it decreases, it's been reduced. Recognizing these changes is key to identifying redox reactions.
For example, consider the reaction of iron with oxygen to form rust (iron oxide): 4Fe + 3O2 → 2Fe2O3. Here, iron (Fe) goes from an oxidation state of 0 to +3, meaning it is oxidized. Oxygen (O2) goes from an oxidation state of 0 to -2, meaning it is reduced. This is a clear redox reaction.
Reactions That Don't Make the Cut: Beyond Electron Transfer
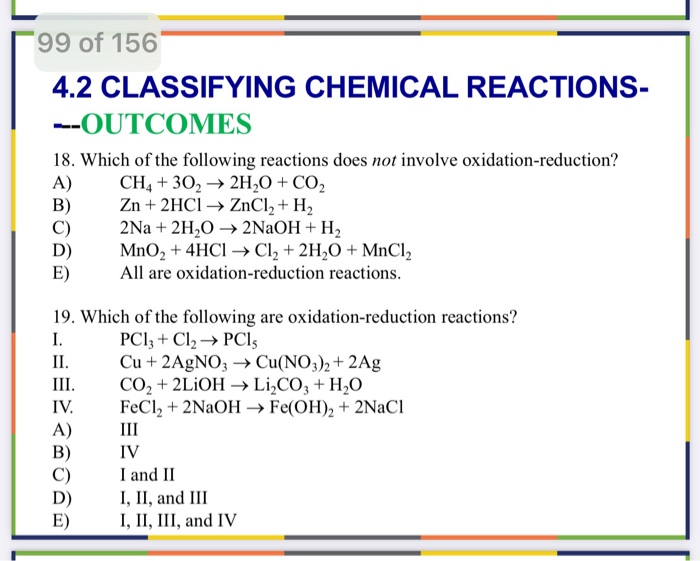
Not all chemical reactions involve electron transfer. Some reactions proceed through different mechanisms, such as acid-base reactions or metathesis reactions, which involve the exchange of ions but not changes in oxidation states.
Let's delve into some common examples of reactions that are not redox reactions.
Acid-Base Reactions: A Proton Shuffle
Acid-base reactions, also known as neutralization reactions, involve the transfer of protons (H+) rather than electrons. When an acid reacts with a base, they neutralize each other, forming a salt and water.
A classic example is the reaction of hydrochloric acid (HCl) with sodium hydroxide (NaOH): HCl + NaOH → NaCl + H2O. In this reaction, the oxidation states of all the atoms remain unchanged. Hydrogen in HCl has an oxidation state of +1, and it remains +1 in H2O. Chlorine in HCl is -1, and in NaCl it is still -1. Similarly, the oxidation states of sodium and oxygen remain constant.
Therefore, acid-base reactions are not classified as redox reactions because they focus on proton transfer, not electron transfer.
Metathesis Reactions: A Partner Swap
Metathesis reactions, also called double displacement reactions, involve the exchange of ions between two reacting compounds. They typically result in the formation of a precipitate, a gas, or a new compound.
A typical example is the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl): AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(aq). In this reaction, silver (Ag) ions combine with chloride (Cl) ions to form solid silver chloride (AgCl), while sodium (Na) ions combine with nitrate (NO3) ions to form sodium nitrate (NaNO3).
Again, the oxidation states of all atoms remain constant. Silver is +1 on both sides, chloride is -1, sodium is +1, and nitrate remains as a polyatomic ion. This signifies that no electron transfer occurred; hence, it's not a redox reaction.
Complex Formation Reactions: A Tight Embrace
Reactions involving the formation of complex ions can sometimes be redox-neutral. Complex ions are formed when a central metal ion is surrounded by ligands (ions or molecules) that donate electron pairs to the metal.
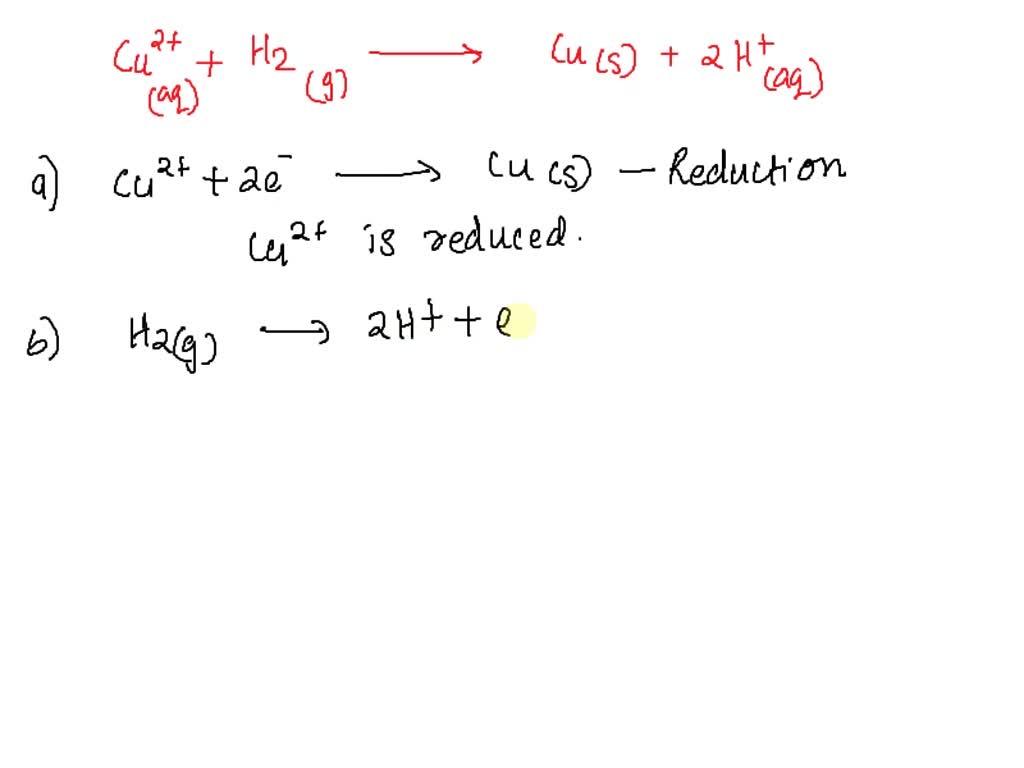
For instance, consider the formation of the tetraamminecopper(II) complex ion: Cu2+(aq) + 4NH3(aq) → [Cu(NH3)4]2+(aq). In this case, the copper ion (Cu2+) coordinates with four ammonia (NH3) molecules. The oxidation state of copper remains +2 throughout the reaction. The ammonia molecules act as ligands, donating electron pairs but not changing the oxidation state of the central copper ion.
While the coordination chemistry is intricate, the absence of electron transfer means this reaction isn't a redox reaction in the strictest sense.
Why Does It Matter? The Significance of Recognizing Reaction Types
Understanding the difference between redox and non-redox reactions is crucial in various fields. In chemistry, it helps predict reaction outcomes and design new chemical processes.
In biology, recognizing redox reactions is fundamental to understanding cellular respiration and photosynthesis. In environmental science, it helps analyze the fate of pollutants and develop remediation strategies.
Furthermore, correctly identifying reaction types is essential in industries like pharmaceuticals and materials science. Accurate classification helps optimize chemical processes, ensure product quality, and minimize environmental impact.
A Final Thought: Appreciating the Diversity of Chemical Reactions
As our group of students in the chemistry lab concludes their whiteboard session, they appreciate the diverse landscape of chemical reactions. While redox reactions play a central role in many natural phenomena, it's equally important to recognize the reactions that proceed through alternative mechanisms.
From acid-base neutralizations to metathesis partner swaps, these non-redox reactions showcase the richness and complexity of chemical transformations. By understanding these different types of reactions, we gain a deeper appreciation for the intricate dance of atoms and molecules that shapes the world around us.
The quest to understand chemical reactions continues, and the insights gained are essential for innovation and progress in various scientific fields. It is a journey of discovery that continually enriches our understanding of the fundamental principles governing matter.
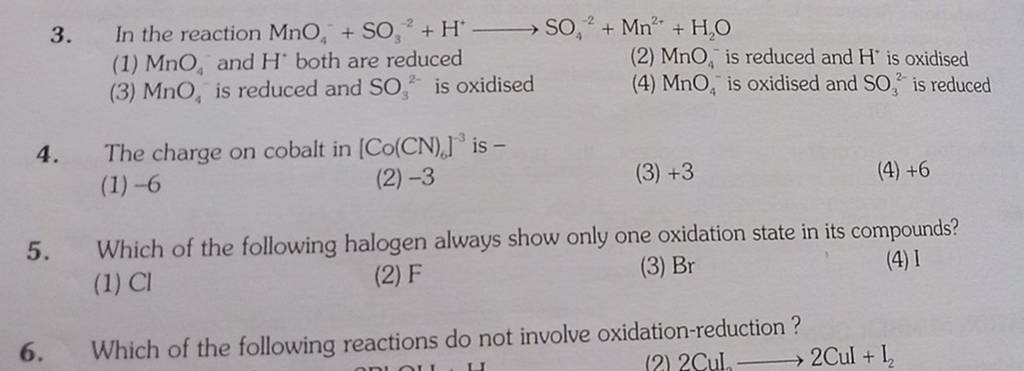
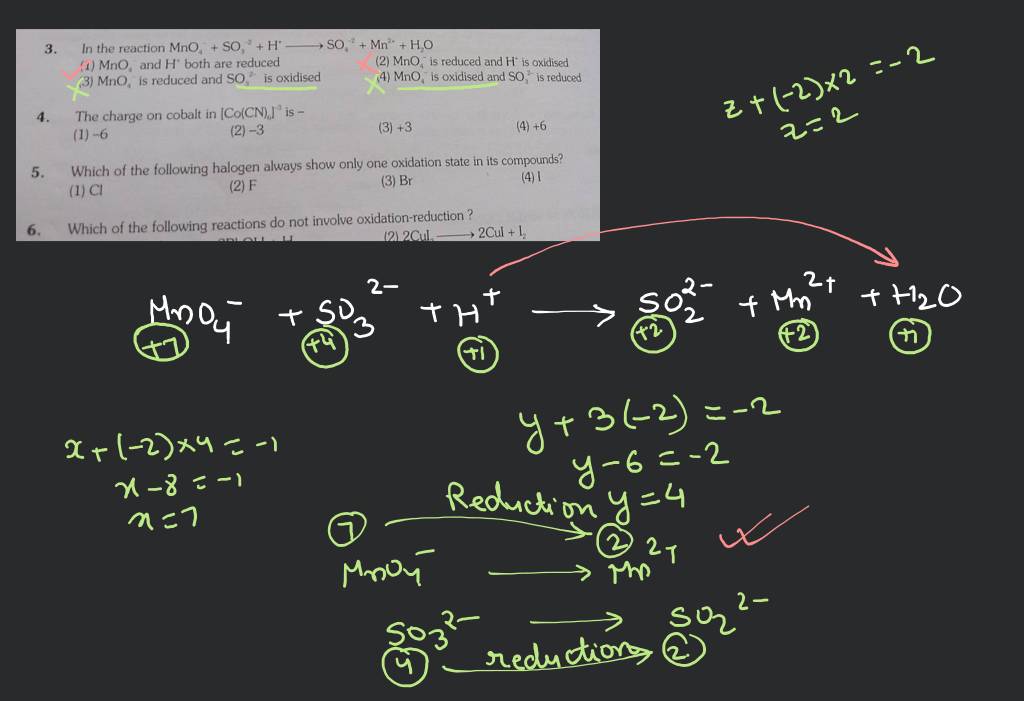
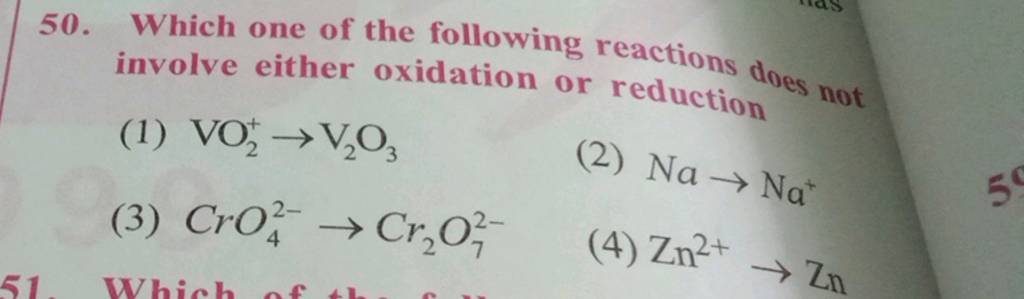




![Which Of The Following Reactions Does Not Involve Oxidation Reduction [Malayalam] Which of the following reactions do not involve oxidation](https://d10lpgp6xz60nq.cloudfront.net/physics_images/BRL_JEE_MN_ADV_CHE_XI_V02_C01_E02_049_Q01.png)

![Which Of The Following Reactions Does Not Involve Oxidation Reduction [Kannada] Which of the following reactions do not involve oxidation re](https://static.doubtnut.com/ss/web/14129298.webp)