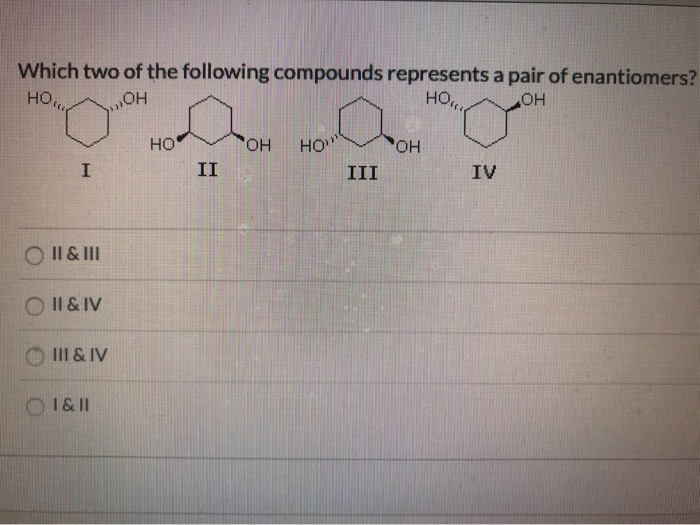
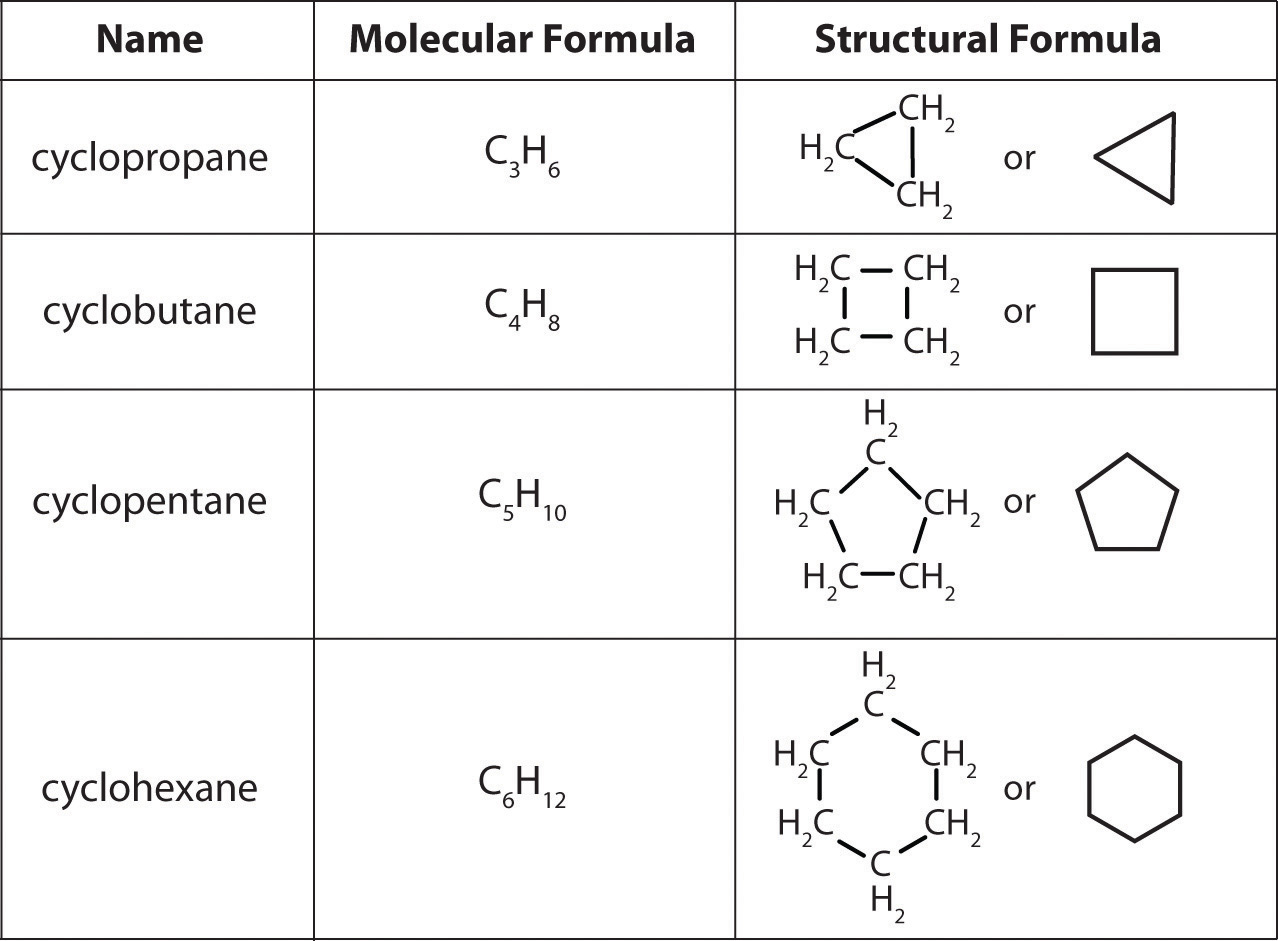
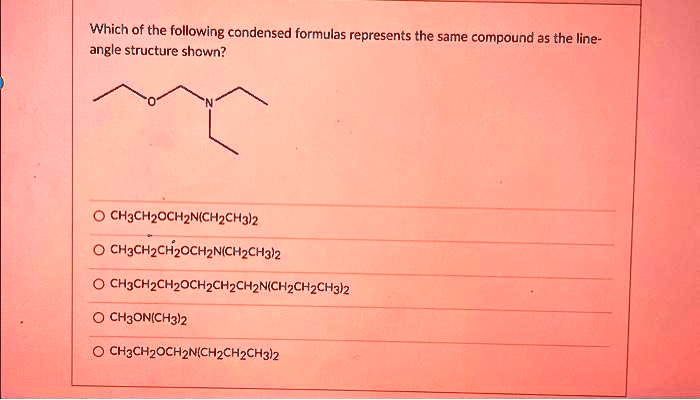
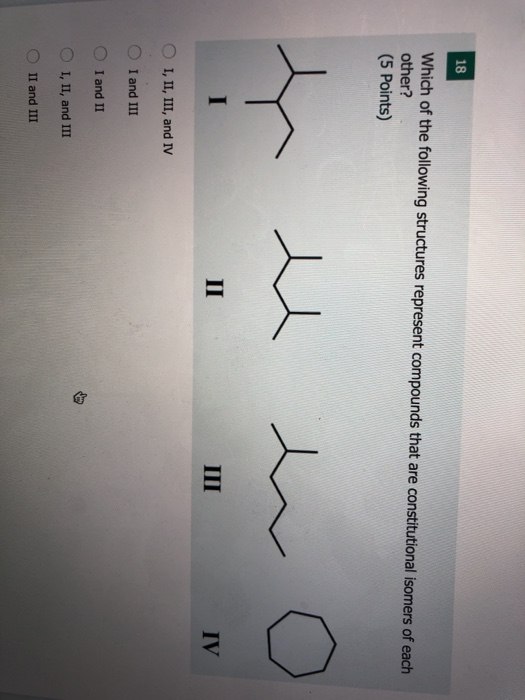
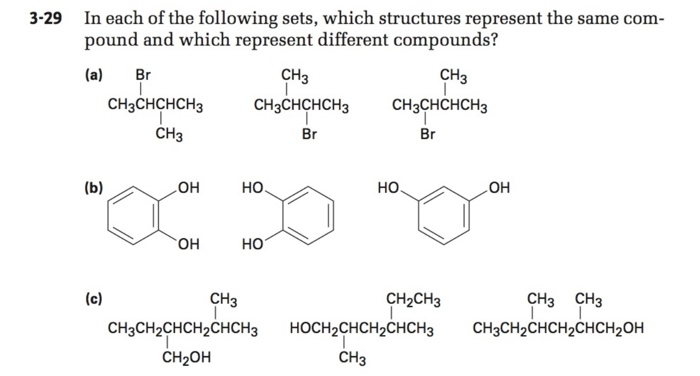
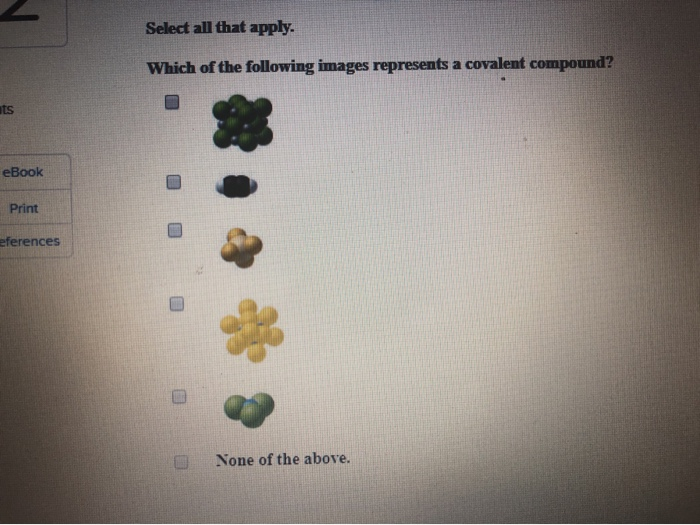
Which Of The Following Represents A Compound
The seemingly simple question, "Which of the following represents a compound?" often unlocks a gateway to understanding the fundamental building blocks of matter. Students, educators, and even seasoned scientists constantly revisit this concept, underscoring its crucial role in chemistry and related fields. Correctly identifying compounds is not merely an academic exercise; it is vital for interpreting chemical reactions, developing new materials, and understanding the very nature of the world around us.
This article delves into the nuances of compound identification, providing clarity and practical examples. The goal is to equip readers with the ability to distinguish between elements, mixtures, and compounds. We will explore common pitfalls and offer strategies for confidently answering this foundational question.
Defining Compounds
A compound is a substance formed when two or more different elements are chemically bonded together in a fixed ratio. This chemical bond is what differentiates a compound from a mixture. The properties of a compound are distinct from the properties of the elements that comprise it.
For example, water (H2O) is a compound formed from hydrogen and oxygen. While hydrogen is a flammable gas and oxygen supports combustion, water is neither flammable nor does it support combustion; it extinguishes fire.
Key Characteristics of Compounds
Compounds are always formed through a chemical reaction. This reaction involves the sharing or transfer of electrons between atoms. This interaction creates a stable arrangement, lower in energy than the individual atoms.
Compounds have a fixed chemical formula. This formula indicates the exact ratio of elements present in the compound. Water's formula, H2O, dictates two hydrogen atoms for every one oxygen atom.
Compounds can only be separated into their constituent elements through chemical reactions. Physical methods, such as filtration or evaporation, will not break the chemical bonds holding the compound together.
Distinguishing Compounds from Mixtures and Elements
The most common error arises from confusing compounds with mixtures. A mixture is a combination of two or more substances that are physically combined, but not chemically bonded. The components of a mixture retain their individual properties and can be separated by physical means.
For example, salt water is a mixture of salt (NaCl) and water (H2O). The salt and water retain their individual properties, and the salt can be recovered by evaporating the water.
An element, on the other hand, is a pure substance consisting of only one type of atom. Elements cannot be broken down into simpler substances by chemical means. Gold (Au) and oxygen (O2) are examples of elements.
Examples to Illustrate the Difference
Consider the following examples: air, sugar (sucrose), iron, and baking soda (sodium bicarbonate). Air is a mixture of gases, primarily nitrogen and oxygen. Iron is an element represented by the symbol Fe.
Sugar (sucrose, C12H22O11) is a compound composed of carbon, hydrogen, and oxygen chemically bonded. Baking soda (sodium bicarbonate, NaHCO3) is also a compound containing sodium, hydrogen, carbon, and oxygen.
To further solidify the understanding, imagine a scenario where a test question provides options like: A) Hydrogen gas (H2), B) Carbon dioxide (CO2), C) A solution of sugar and water, D) Liquid nitrogen. The correct answer would be B, carbon dioxide, as it's the only chemically bonded substance.
Common Pitfalls and How to Avoid Them
A common misconception is that any combination of elements is a compound. Remember, the elements must be chemically bonded. Simply mixing them together does not create a compound.
Another pitfall is failing to recognize chemical formulas. Familiarize yourself with common chemical symbols and the basic rules of chemical nomenclature. For instance, knowing that NaCl represents sodium chloride (table salt) will instantly identify it as a compound.
Paying attention to the phases (solid, liquid, gas) can also provide clues. While mixtures can exist in any phase, some compounds are more commonly encountered in a particular phase at room temperature and pressure. However, this is not a definitive rule, and one should consider the chemical composition primarily.
The Importance of Understanding Compounds
Identifying compounds accurately is fundamental to numerous scientific disciplines. In medicine, understanding the chemical structure of drugs is crucial for understanding how they interact with the body. In materials science, designing new materials with specific properties relies on understanding the compounds that make them up.
In environmental science, identifying pollutants and understanding their chemical behavior is essential for developing effective remediation strategies.
"A thorough understanding of chemical compounds forms the cornerstone of scientific innovation and environmental responsibility,"emphasizes Dr. Emily Carter, a renowned chemist at Princeton University.
Ultimately, mastering the concept of compounds allows one to better understand the world at a molecular level. This understanding empowers informed decision-making in many different contexts.
Looking Ahead: Future Directions in Compound Research
The study of compounds is constantly evolving with advances in technology and scientific understanding. Researchers are continually discovering new compounds with unique properties, such as novel pharmaceuticals or materials with enhanced strength or conductivity.
Computational chemistry plays an increasing role in predicting the properties of new compounds before they are even synthesized in the laboratory. This accelerates the discovery process and reduces the need for costly and time-consuming experiments.
As our understanding of compounds deepens, we can expect to see even more transformative innovations in fields ranging from medicine to energy to environmental protection. Compounds are the literal components of advancement.