Which Of These Causes The Release Of Neurotransmitter Molecules

The human brain, a universe contained within our skulls, relies on a complex symphony of electrochemical signals to function. Disruptions in this signaling process can lead to a cascade of neurological disorders, underscoring the critical importance of understanding the precise mechanisms that govern neurotransmitter release.
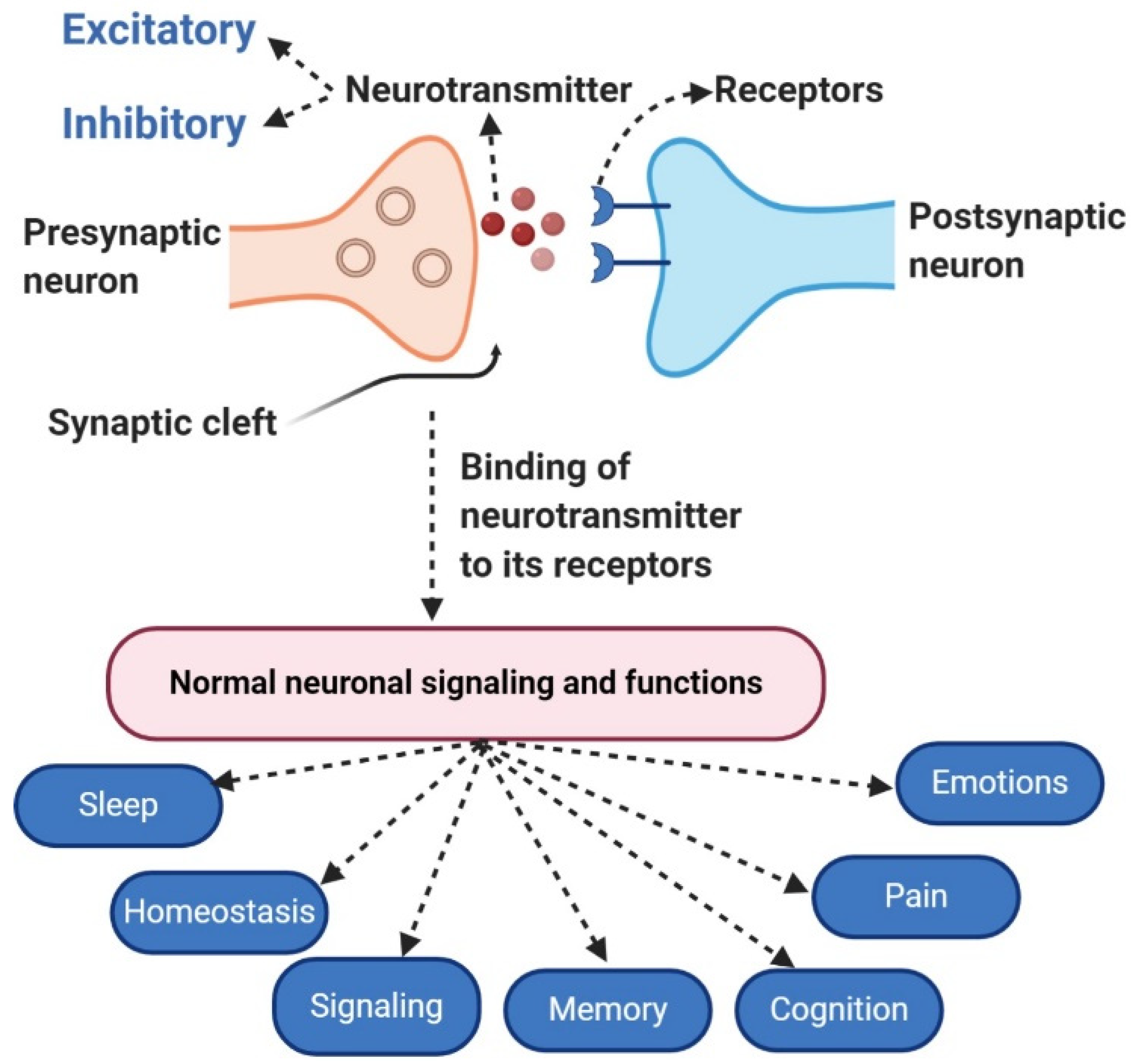
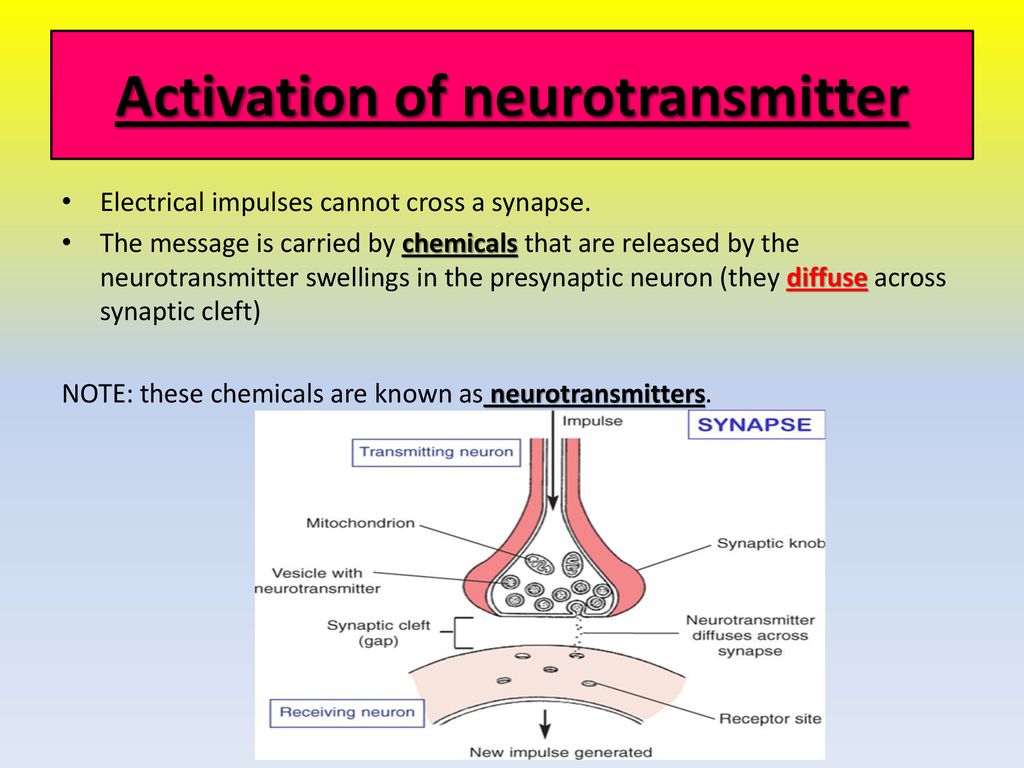
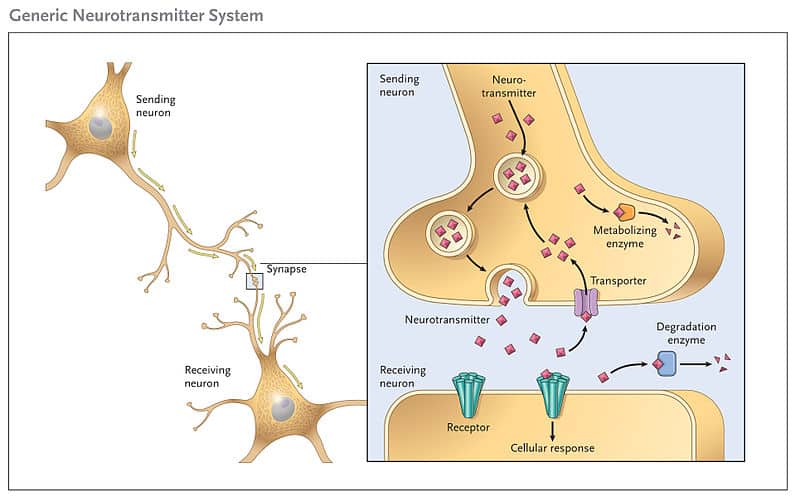
The release of neurotransmitter molecules, the chemical messengers that transmit signals between neurons, is a cornerstone of neural communication. This intricate process, triggered by a specific event, dictates everything from our thoughts and emotions to our movements and bodily functions. So, the question is, what precisely causes this release? This article delves into the scientific consensus surrounding the key trigger for neurotransmitter exocytosis, exploring the intricate dance of ions, proteins, and vesicles that underlies this fundamental biological process.
The Prime Mover: Calcium Influx
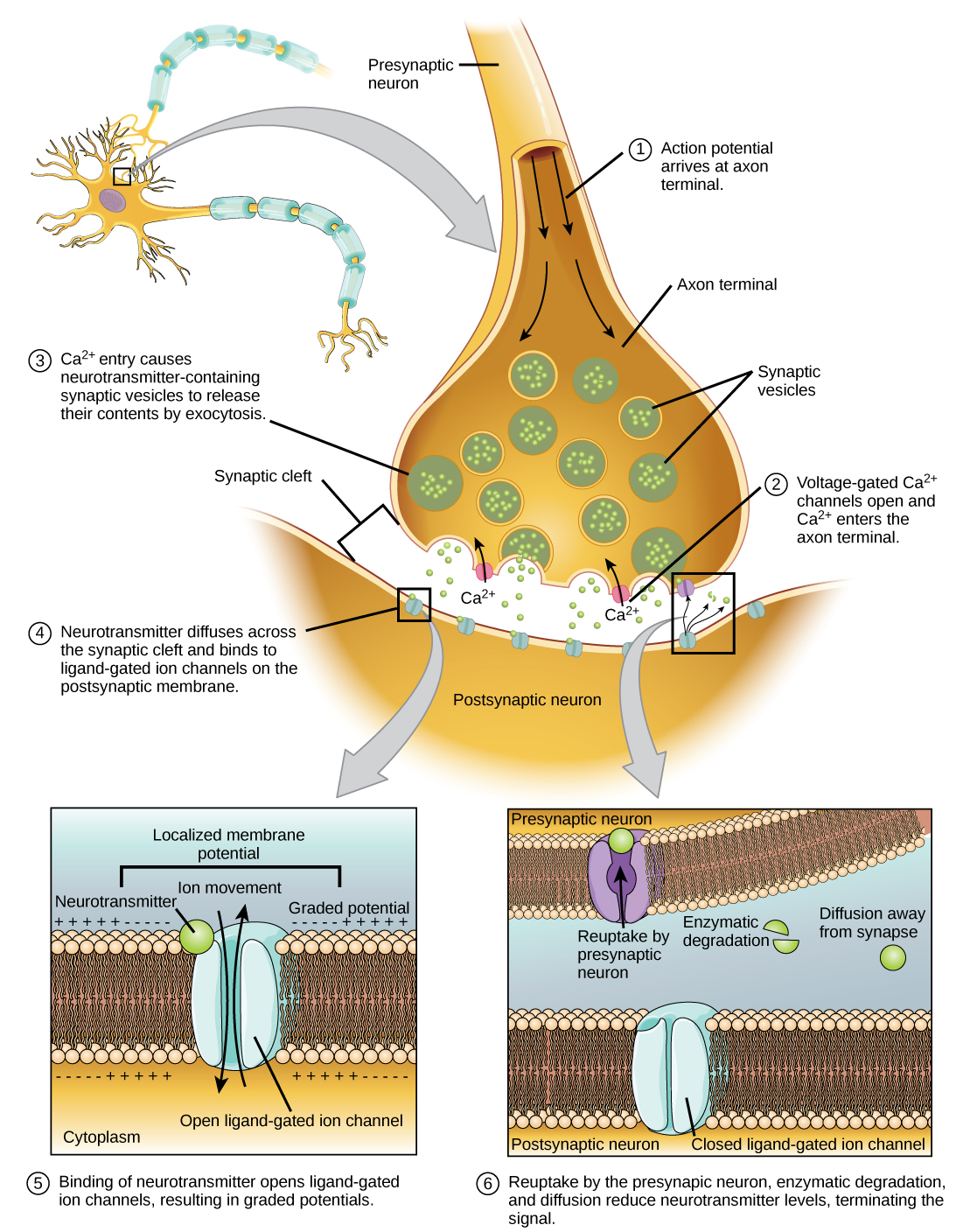
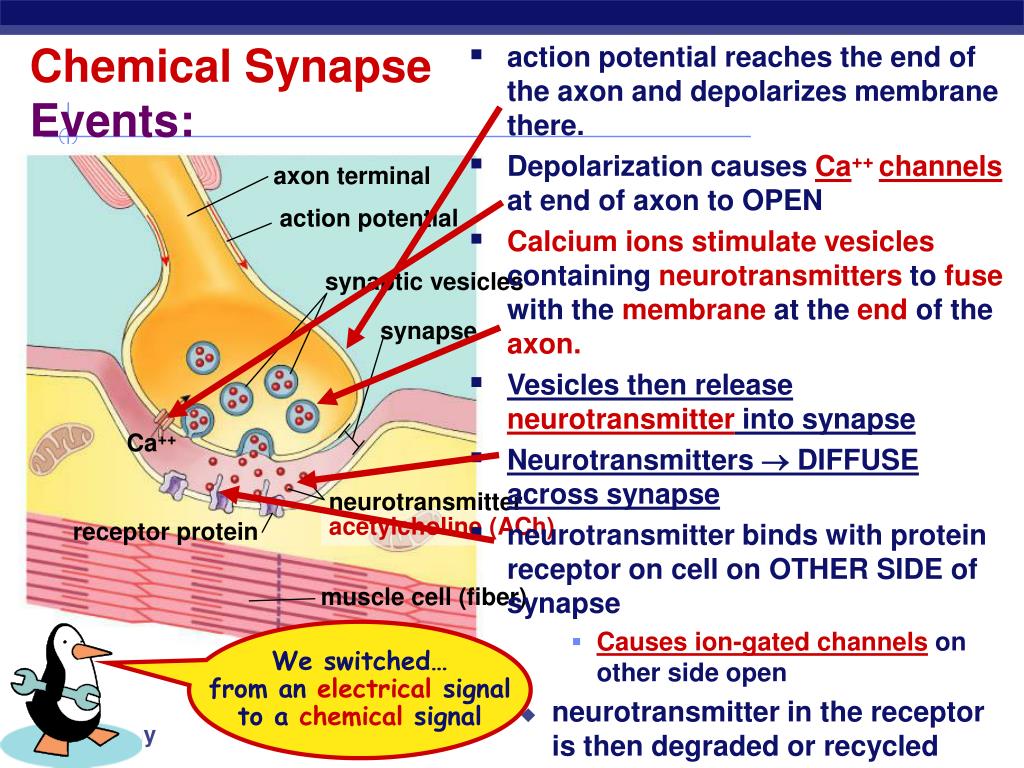
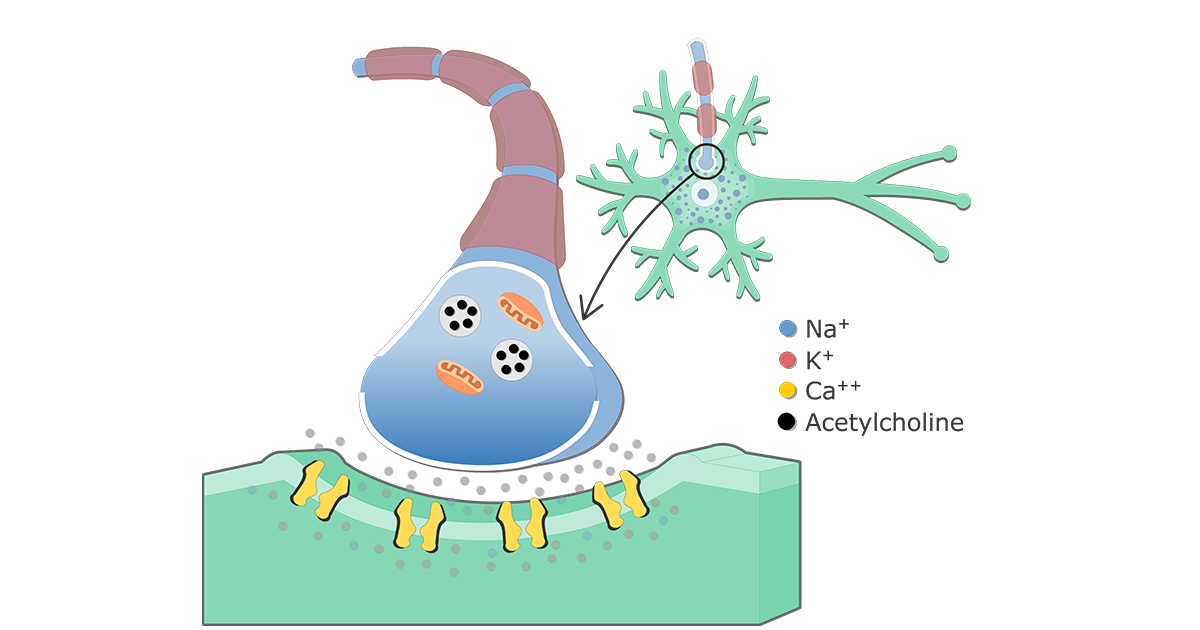
The primary trigger for neurotransmitter release is the influx of calcium ions (Ca2+) into the presynaptic terminal, the end of the neuron that sends the signal.
This influx is not a spontaneous event but is tightly controlled and directly linked to the arrival of an action potential, the electrical signal that travels down the neuron.
According to the National Institutes of Health (NIH), the depolarization of the presynaptic terminal membrane caused by the action potential opens voltage-gated calcium channels.
Voltage-Gated Calcium Channels: Gatekeepers of Neurotransmission
Voltage-gated calcium channels are specialized protein structures embedded in the presynaptic terminal membrane. They remain closed at the resting membrane potential but open rapidly when the membrane becomes depolarized, a key aspect highlighted in numerous neuroscience textbooks and research papers.
This opening allows a rapid influx of calcium ions down their electrochemical gradient, flowing from the extracellular space, where calcium concentration is high, into the presynaptic terminal, where calcium concentration is normally low.
The precise types of calcium channels involved can vary depending on the type of neuron and the specific synapse, but the principle remains the same: depolarization-induced opening leading to calcium influx.
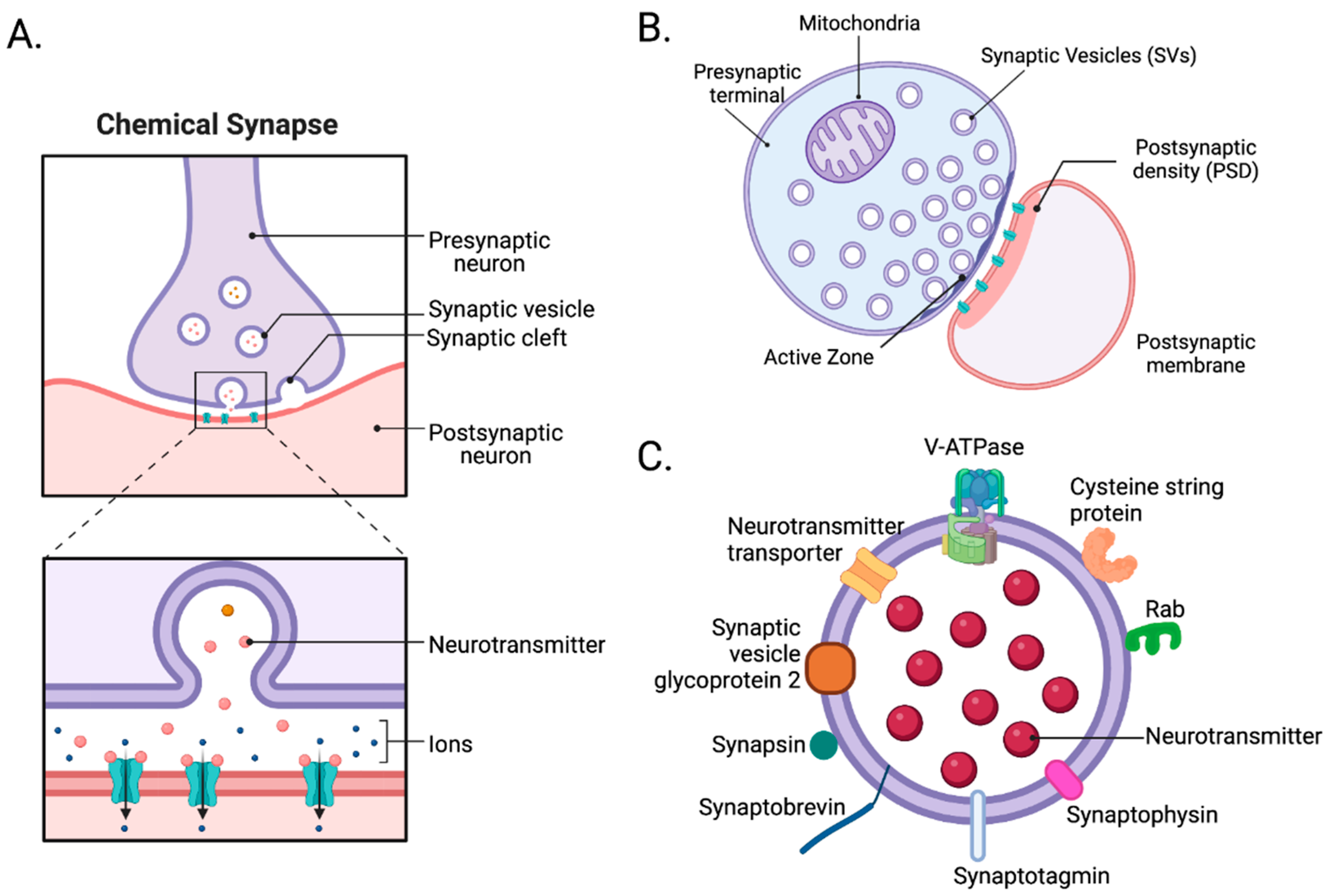
The Role of SNARE Proteins
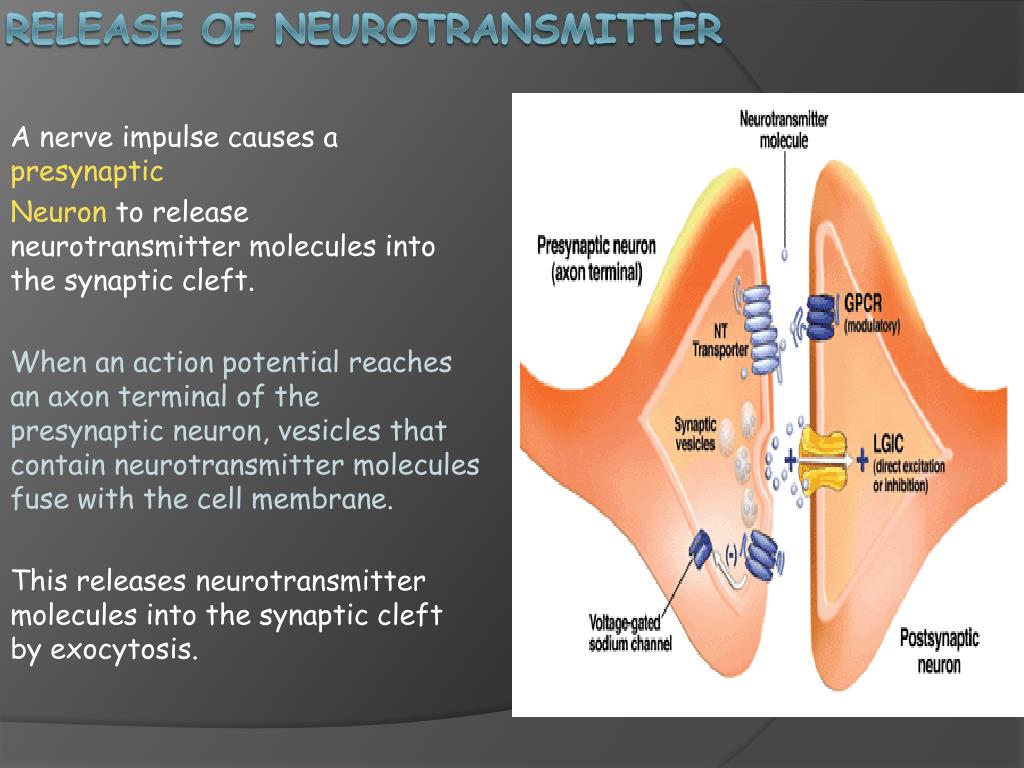
Once inside the presynaptic terminal, calcium ions don't directly release neurotransmitters.
Instead, they bind to a protein called synaptotagmin, which is associated with synaptic vesicles.
These vesicles contain the neurotransmitter molecules, packaged and ready for release. Synaptotagmin is a calcium sensor.
This crucial event initiates a cascade of protein-protein interactions involving the SNARE proteins (Soluble NSF Attachment protein Receptor). These proteins, VAMP, syntaxin, and SNAP-25, form a complex that physically tethers the synaptic vesicle to the presynaptic membrane.
Calcium binding to synaptotagmin causes a conformational change in the SNARE complex, essentially "zipping" the vesicle and membrane together.
This fusion creates a pore through which the neurotransmitter molecules are expelled into the synaptic cleft, the space between the presynaptic and postsynaptic neurons.
This complex process is described in detail by the Society for Neuroscience, with diagrams illustrating the molecular interactions involved.
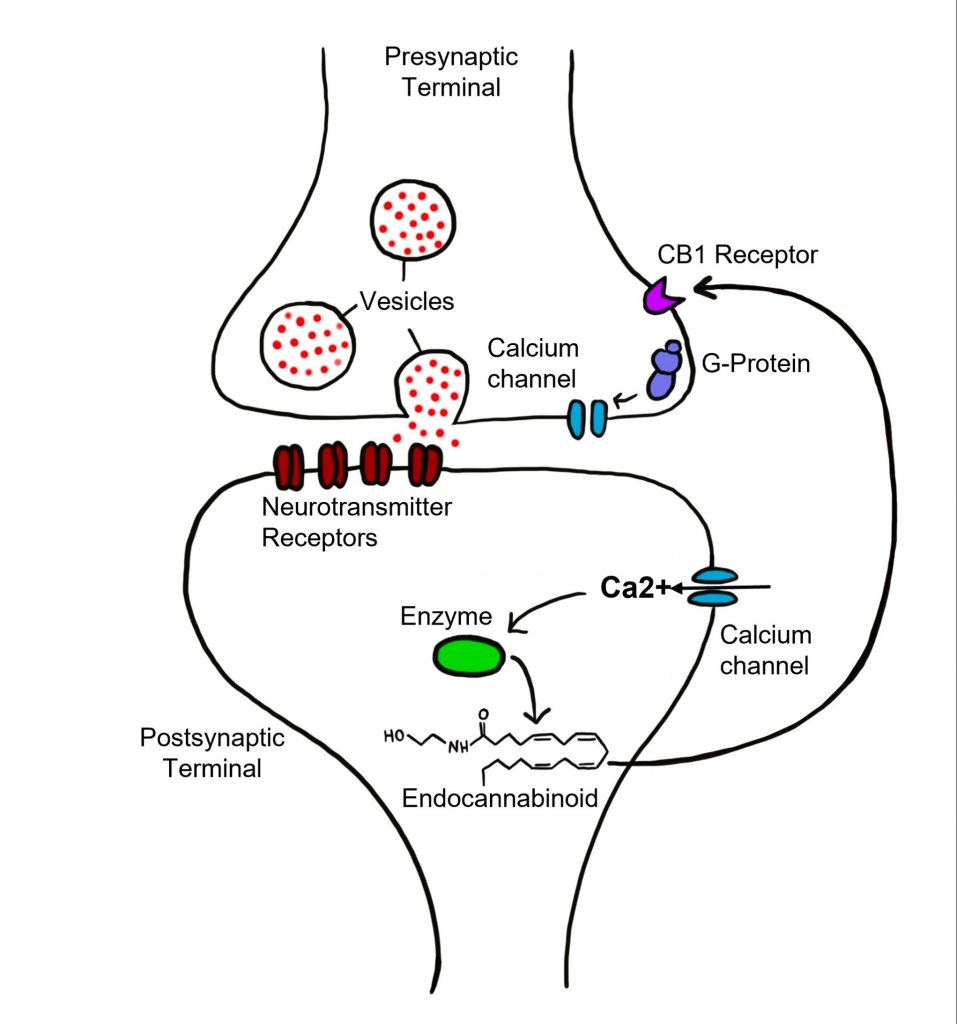
Beyond Calcium: Modulation and Fine-Tuning
While calcium influx is the primary trigger, the process of neurotransmitter release is not simply an on/off switch.
Other factors can modulate the amount of neurotransmitter released, including the frequency and duration of action potentials, the presence of other ions, and the activity of presynaptic receptors.
For instance, some neurotransmitters can act on presynaptic receptors to inhibit calcium channel activity, providing a feedback mechanism to regulate their own release. Studies presented in the journal Neuron show that neuromodulators like dopamine and serotonin can influence neurotransmitter release by altering the sensitivity of calcium channels.
Furthermore, the distance between the calcium channels and the synaptotagmin molecules influences the efficiency of release.
Vesicles located closer to the calcium channels are more likely to fuse and release their contents upon calcium influx.
Implications for Neurological Disorders
The precise control of neurotransmitter release is vital for normal brain function.
Dysfunction in any aspect of this process, from calcium channel abnormalities to defects in SNARE proteins, can contribute to a range of neurological disorders.
For example, mutations in genes encoding voltage-gated calcium channels have been linked to certain types of epilepsy and migraine. The National Institute of Neurological Disorders and Stroke (NINDS) provides resources on these disorders and their potential genetic causes.
Similarly, disruptions in neurotransmitter release are implicated in neurodegenerative diseases such as Alzheimer's and Parkinson's disease, as well as psychiatric disorders like schizophrenia and depression.
Future Directions and Therapeutic Potential
Understanding the molecular mechanisms of neurotransmitter release is an active area of research.
Scientists are continuously exploring new ways to target this process for therapeutic intervention.
For example, drugs that modulate calcium channel activity are already used to treat conditions such as epilepsy and chronic pain. Further research into the specific calcium channel subtypes involved in different neurological disorders could lead to the development of more targeted and effective therapies.
Moreover, researchers are investigating the potential of gene therapy to correct defects in proteins involved in neurotransmitter release.
This approach could offer a long-term solution for patients with genetic disorders affecting synaptic function.
The knowledge gained from these studies promises to unlock new avenues for treating a wide range of neurological and psychiatric disorders.