Which Property Is True For Metals

Urgent warnings are circulating as a critical evaluation of metallic properties reveals a widespread misunderstanding among students. The correct identification of properties, particularly regarding conductivity and malleability, is crucial for multiple engineering and scientific applications.
This report aims to clarify the essential properties of metals, debunking common misconceptions and reinforcing fundamental scientific knowledge. Accurate understanding of these characteristics is vital for both academic success and practical application in various industries.
Defining Metallic Properties
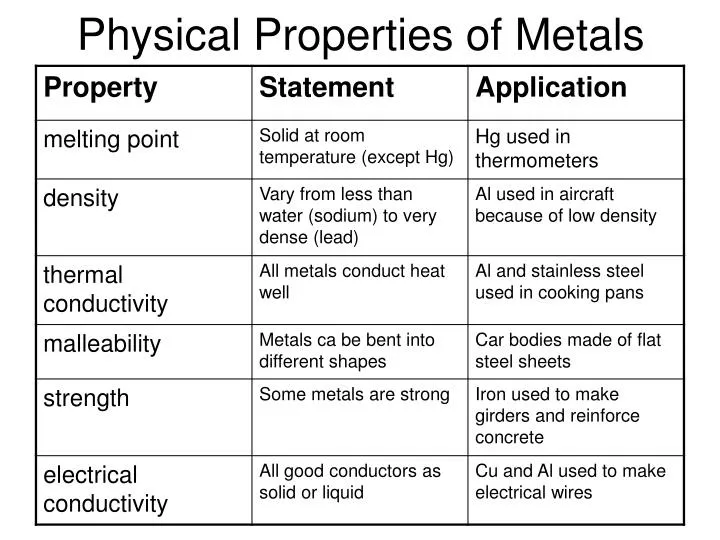
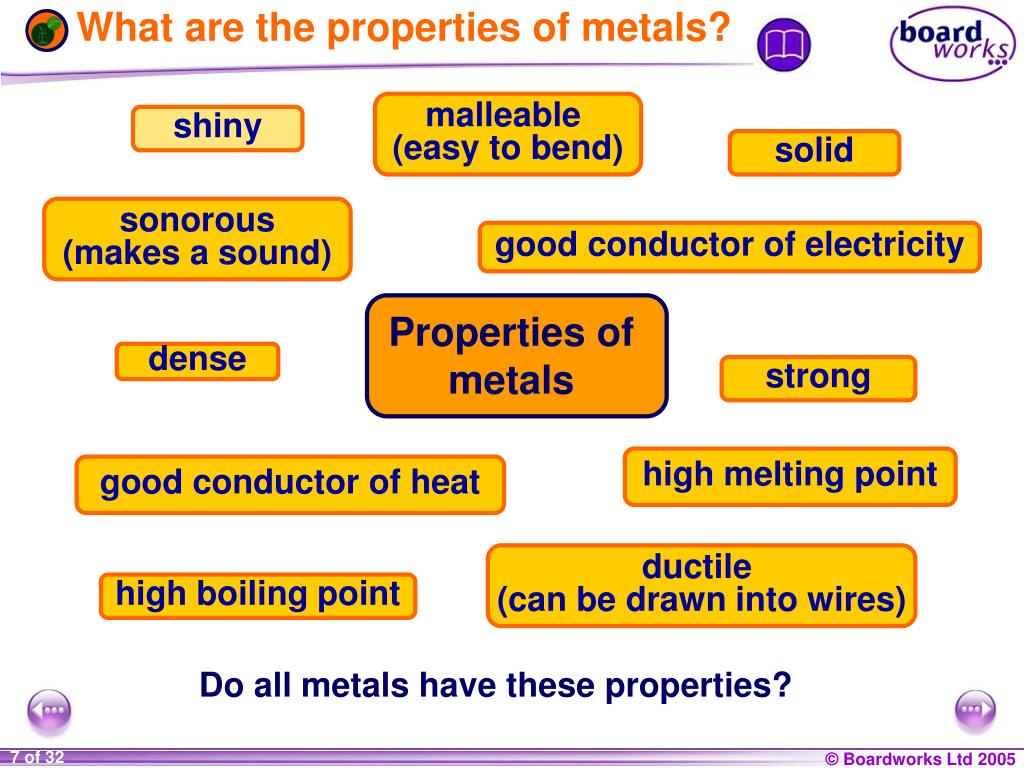
Metals are fundamentally characterized by several key properties. These include high electrical and thermal conductivity, malleability, ductility, and a lustrous appearance.
Electrical conductivity stems from the free movement of electrons within the metal's structure. Thermal conductivity is closely linked, allowing metals to efficiently transfer heat.
Essential Properties Explained
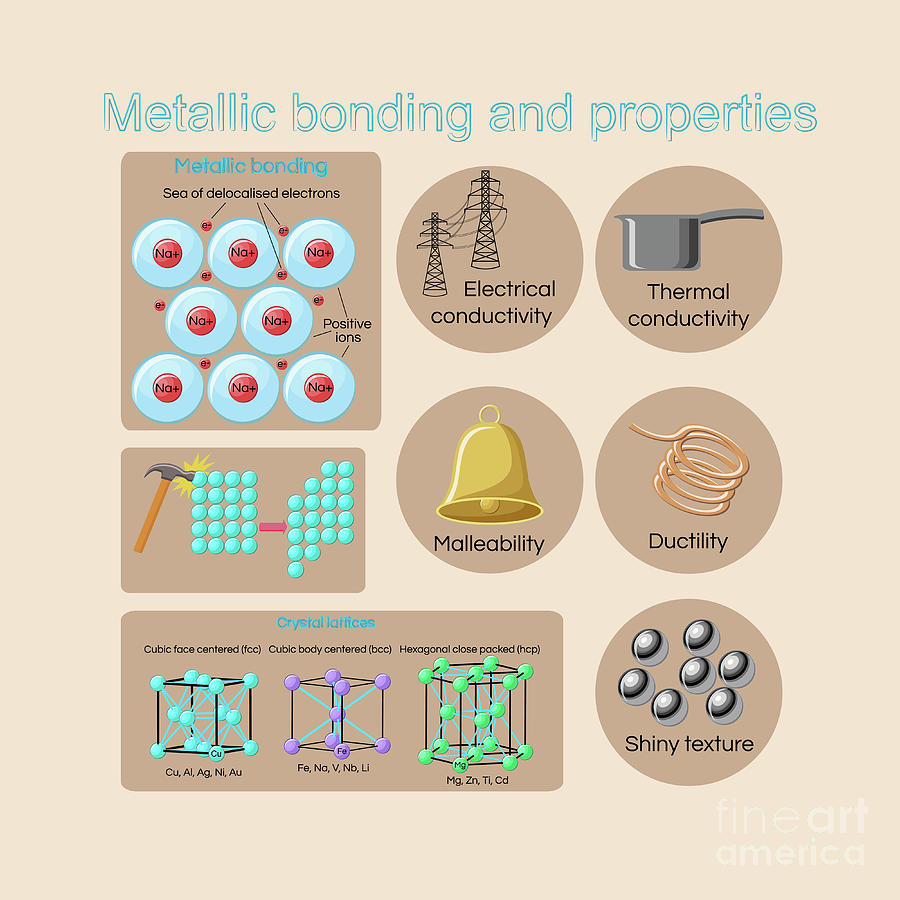
Electrical Conductivity: Metals allow electricity to flow easily due to their electron arrangement. Electrons are not bound to individual atoms but exist in a "sea" of electrons, facilitating charge transfer.
Thermal Conductivity: Metals transfer heat efficiently as the free electrons also facilitate heat energy transfer. This makes them ideal for applications like heat sinks and cooking utensils.
Malleability: Metals can be hammered or pressed into different shapes without breaking. This characteristic is essential for manufacturing processes.
Ductility: Metals can be drawn into wires. Copper, for example, is widely used for electrical wiring due to its ductility and conductivity.
Luster: Metals exhibit a shiny surface due to their ability to reflect light. This reflective property is used in decorative and functional applications.
Addressing Misconceptions
Common misconceptions often involve confusing metallic properties with those of other materials. For example, assuming all shiny materials are metals is incorrect.
Similarly, misunderstanding the role of electrons in conductivity leads to incorrect conclusions about metal behavior. It's important to remember the "sea" of electrons model.
Another misconception is the idea that all metals are hard and strong. Some metals, such as sodium and potassium, are quite soft.
The Importance of Accurate Knowledge
Correct identification of metallic properties is crucial in fields such as engineering and materials science. Selecting the appropriate material for a specific application depends on understanding these properties.
For example, in electrical engineering, choosing the right metal for wiring relies on knowing its conductivity and resistance. In construction, the strength and malleability of metals determine their suitability for structural components.
In chemistry, understanding how metals react based on their electron configuration is essential for synthesizing new compounds and understanding chemical processes.
Confirmed Facts About Metals
Scientific data confirms that metals are characterized by a specific electron configuration. This electron configuration enables the characteristic properties of conductivity, malleability, and ductility.
Research consistently demonstrates that these properties are intrinsic to the metallic bond. The metallic bond involves the sharing of electrons across a lattice of atoms.
Experiments have shown that altering the metallic structure can change these properties. Adding impurities, for instance, can decrease conductivity.
"Metals are essential building blocks of modern technology, and understanding their fundamental properties is paramount," stated Dr. Emily Carter, a leading materials scientist at MIT.
Ongoing Developments and Next Steps
Ongoing research focuses on developing new alloys with enhanced properties. Scientists are exploring ways to improve the strength, conductivity, and corrosion resistance of metals.
Further investigation into nanoscale metallic structures is revealing novel applications. Nanomaterials exhibit unique properties compared to their bulk counterparts.
Educational initiatives are being implemented to address the misconceptions about metallic properties. Workshops and online resources are being developed to enhance understanding.
Universities and research institutions are collaborating to improve materials science education. They seek to provide students with a solid foundation in metallic properties and their applications.
Efforts are also underway to promote critical thinking skills. Students should be able to differentiate between accurate information and misinformation about materials.