Which Proteins Are Marked For Destruction
Imagine a bustling metropolis, but instead of cars and people, it's a city of proteins. Some are busy building structures, others are transporting goods, and some are even involved in communication. But amidst this vibrant activity, there's a silent cleanup crew, constantly identifying and dismantling proteins that are old, damaged, or simply no longer needed. This continuous process of protein degradation is essential for maintaining cellular health, ensuring that the city, our cells, doesn't become overwhelmed with debris.
At the heart of this cellular sanitation system lies the question: Which proteins are tagged for destruction, and how is this process regulated? Understanding this intricate mechanism is crucial because it plays a pivotal role in everything from immune responses and cell growth to preventing diseases like cancer and neurodegeneration. This article delves into the fascinating world of protein degradation, exploring the key players and the sophisticated signals that determine a protein's fate.
The Ubiquitin-Proteasome System: The Cell's Recycling Center
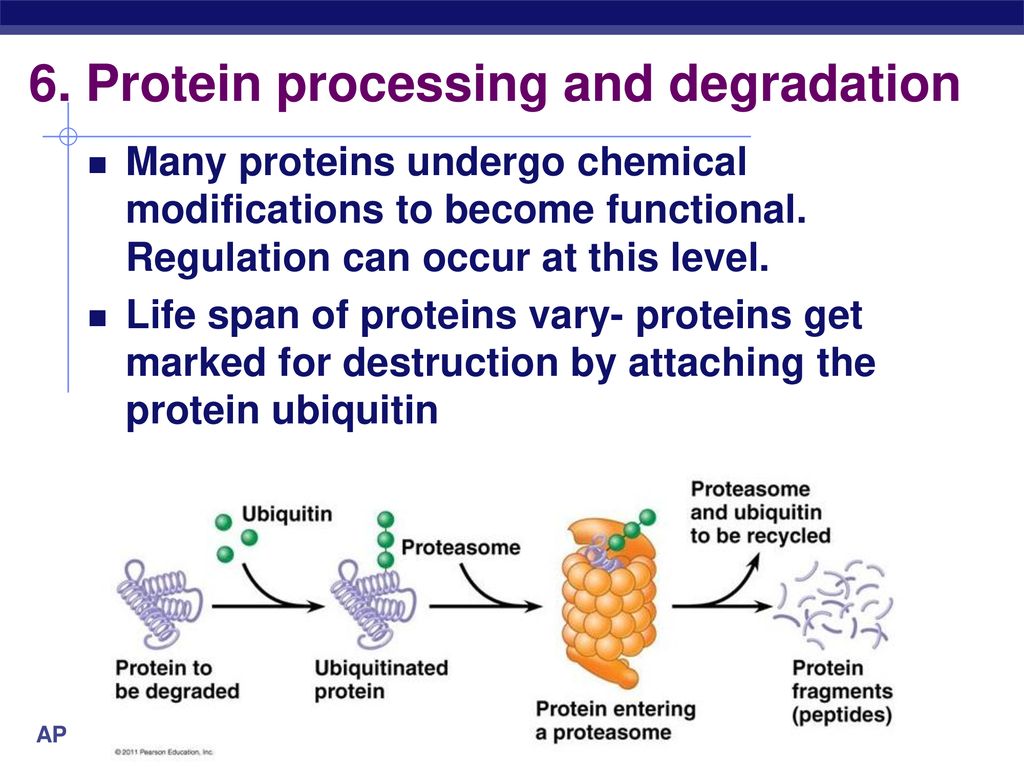
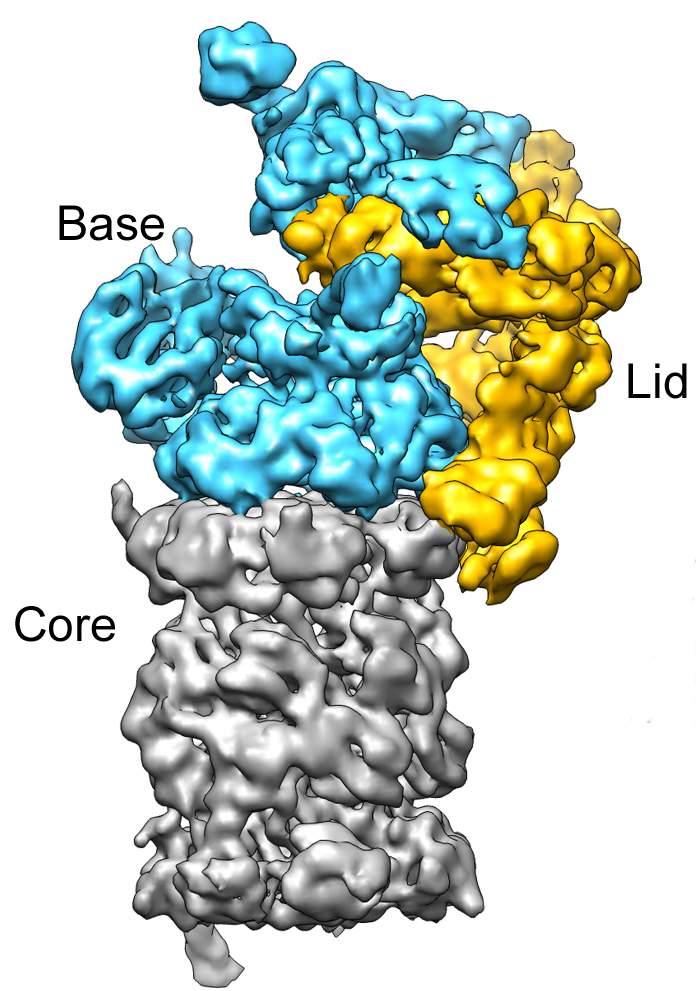
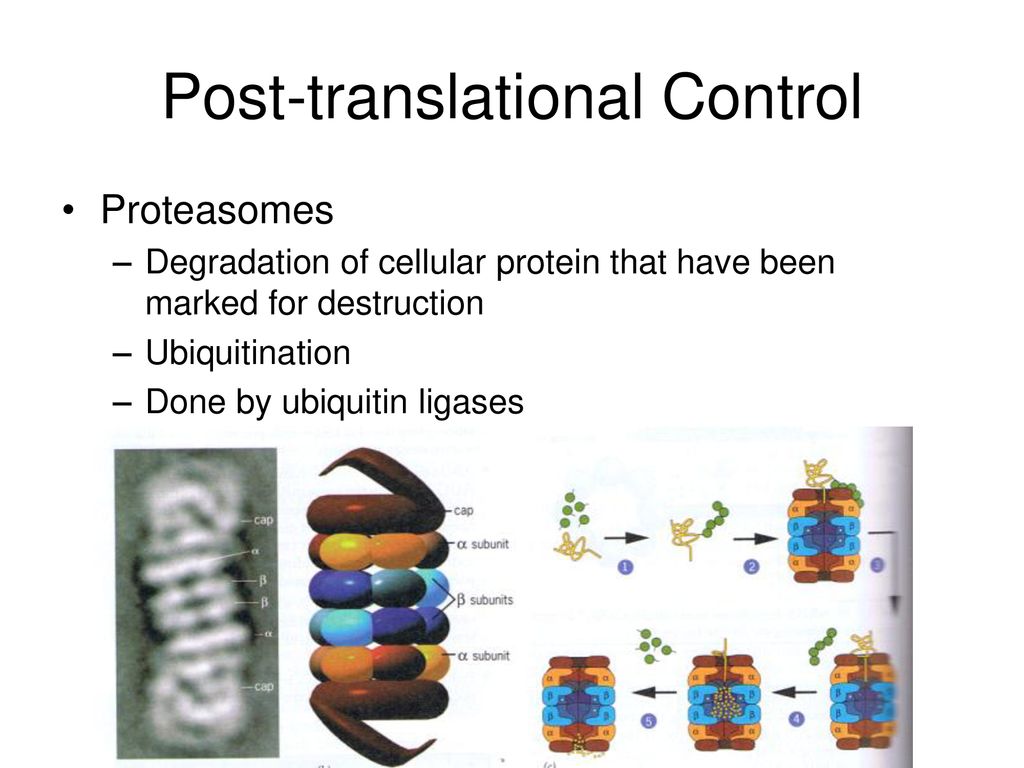
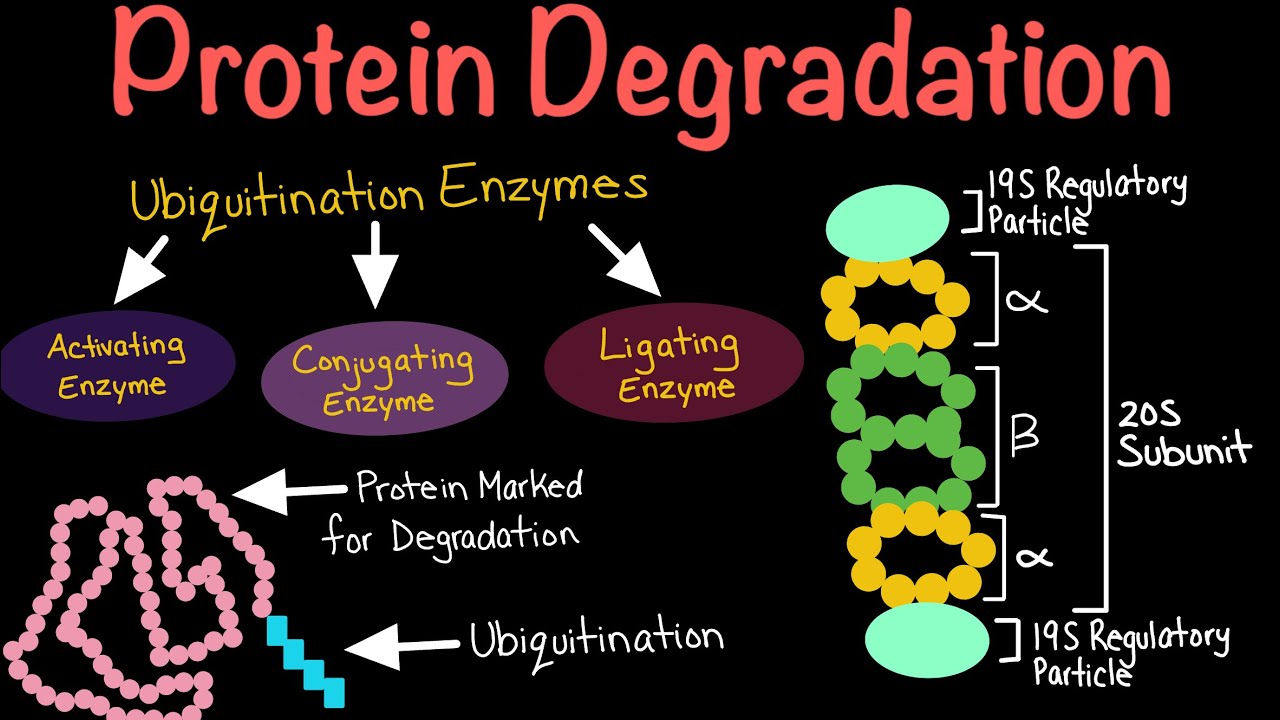
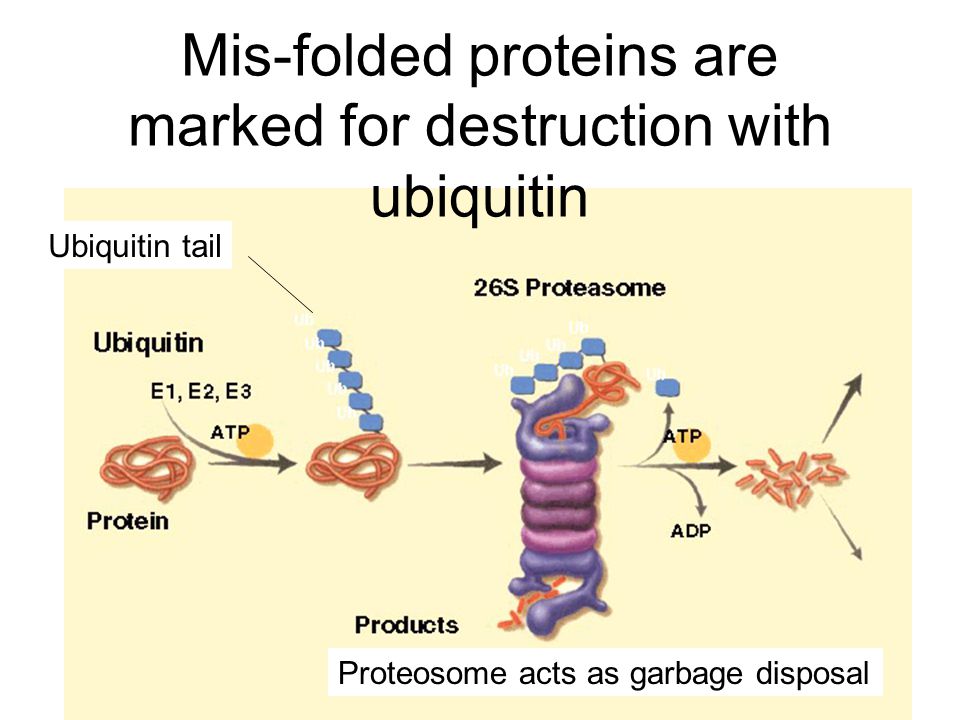
The primary pathway for protein degradation in our cells is the ubiquitin-proteasome system (UPS). Think of the proteasome as the cell's recycling center, a massive protein complex that breaks down unwanted proteins into smaller peptides. But before a protein can enter the proteasome, it needs a special tag: ubiquitin.
Ubiquitin is a small protein that acts like a molecular "kiss of death." The process of attaching ubiquitin to a target protein is called ubiquitylation, and it's a complex, multi-step process involving a cascade of enzymes: E1 ubiquitin-activating enzymes, E2 ubiquitin-conjugating enzymes, and E3 ubiquitin ligases. It’s like a highly specialized labeling system for cellular waste management.
E3 Ubiquitin Ligases: The Gatekeepers of Protein Degradation
The E3 ubiquitin ligases are arguably the most important players in this system. They are the gatekeepers that determine which proteins get ubiquitylated and, therefore, marked for destruction. There are hundreds of different E3 ligases in human cells, each recognizing a specific set of target proteins.
This specificity is what allows the cell to precisely control protein turnover. These enzymes are the master selectors in the cellular recycling program.
One well-studied example is the SCF (Skp1-Cullin-F-box protein) complex, a family of E3 ligases that regulate a wide range of cellular processes, including cell cycle progression and DNA replication. The F-box protein component of the SCF complex determines the specific target protein to be ubiquitylated.
Another crucial E3 ligase is APC/C (Anaphase-Promoting Complex/Cyclosome), which plays a critical role in regulating the cell cycle, specifically the transition from metaphase to anaphase. APC/C targets proteins like securin and cyclin B for degradation, allowing sister chromatids to separate and the cell to divide. In simple terms, APC/C makes sure the cell divides properly.
Signals for Destruction: Deciphering the Code
So, how do E3 ligases "know" which proteins to target? The answer lies in specific signals or motifs within the target proteins themselves. These signals can be thought of as molecular "danger flags" that alert the E3 ligases.
These signals can be diverse, but some common ones include: N-terminal degrons, which are specific amino acid sequences at the beginning of the protein; phosphorylation sites, where phosphate groups are added to the protein; and oxidation sites, which indicate damage caused by reactive oxygen species. These are all molecular whispers communicating a protein's need for recycling.
For example, the N-end rule pathway recognizes proteins with specific amino acids at their N-terminus (the beginning of the protein). These amino acids act as degrons, signaling the protein to be degraded by the UPS. This is like having a universal tag that E3 ligases immediately recognize.
Another interesting example involves misfolded proteins. When proteins fail to fold correctly, they can expose hydrophobic regions that are normally buried inside the protein structure. These exposed regions can be recognized by E3 ligases, leading to ubiquitylation and degradation. The cell has ways of detecting and eliminating these faulty structures.
The Role of Protein Degradation in Health and Disease
The UPS is not just a cellular cleanup crew; it's a vital regulator of many essential cellular processes. When the UPS malfunctions, it can lead to a variety of diseases. This system isn't just cleaning; it's crucial for maintaining health.
Cancer is often associated with dysregulation of the UPS. For example, mutations in E3 ligases can lead to the accumulation of proteins that promote cell growth and division, contributing to tumor formation. Furthermore, some cancer cells develop resistance to chemotherapy by upregulating the UPS, allowing them to degrade the drugs designed to kill them. It is a double-edged sword - cancer can both cause and exploit disruptions in the UPS.
Neurodegenerative diseases, such as Alzheimer's and Parkinson's, are also linked to impaired protein degradation. In these diseases, misfolded proteins accumulate and form toxic aggregates, disrupting neuronal function. A malfunctioning UPS can exacerbate this process by failing to clear these aggregates. These diseases highlight the importance of proper protein management in the brain.
The UPS also plays a crucial role in immune responses. It is involved in processing antigens and presenting them on the surface of immune cells, allowing the immune system to recognize and respond to pathogens. Disruption of the UPS can impair the immune response, making individuals more susceptible to infections.
"Understanding the intricacies of protein degradation is fundamental to developing new therapies for a wide range of diseases." - Dr. Emily Carter, Lead Researcher at the Institute of Cellular Biology.
Future Directions: Targeting the UPS for Therapeutic Intervention
Given the critical role of the UPS in health and disease, it has become a major target for drug development. Researchers are exploring ways to manipulate the UPS to treat a variety of conditions. Scientists are eager to tap into its potential for therapeutic benefits.
One approach is to develop drugs that inhibit the proteasome. These drugs can be effective in treating certain cancers, such as multiple myeloma, where the proteasome is overactive. For instance, bortezomib is a proteasome inhibitor that is widely used in the treatment of multiple myeloma. However, proteasome inhibitors can have significant side effects, as they disrupt the normal protein turnover processes in healthy cells.
Another approach is to develop drugs that target specific E3 ligases. This would allow for more precise control over protein degradation, reducing the risk of off-target effects. Researchers are actively working to identify and characterize E3 ligases that are involved in specific diseases, with the goal of developing targeted therapies.
PROTACs (Proteolysis-Targeting Chimeras) are a promising new class of drugs that work by recruiting E3 ligases to target proteins of interest. PROTACs are bifunctional molecules, with one end binding to the target protein and the other end binding to an E3 ligase. This brings the target protein into close proximity with the E3 ligase, leading to ubiquitylation and degradation. They essentially act as molecular matchmakers, bringing together targets and the degradation machinery.
Conclusion: A Continuous Cycle of Renewal
The world of protein degradation is a complex and dynamic one. It's a continuous cycle of renewal, ensuring that our cells remain healthy and functional. By understanding which proteins are marked for destruction and how this process is regulated, we can gain valuable insights into the fundamental mechanisms of life and develop new strategies for treating diseases.
While we've only scratched the surface of this intricate system, it's clear that the UPS is far more than just a cellular garbage disposal. It's a sophisticated regulatory network that plays a critical role in maintaining cellular homeostasis. Further research in this area promises to unlock new opportunities for improving human health and combating disease. As we continue to unravel the mysteries of protein degradation, we can look forward to a future where we have the power to manipulate this fundamental process for therapeutic benefit.

+have+a+destruction+box+sequence+recognized+by+a+domain+of+the+corresponding+Ubiquitin+Ligase..jpg)