Which Statement Describes The Distribution Of Charge In An Atom

For decades, the understanding of atomic structure has been fundamental to scientific progress, shaping fields from medicine to materials science. One of the core questions in this realm revolves around the distribution of charge within an atom, a concept that continues to be refined and explored by researchers worldwide.
The core debate centers on accurately describing how positive and negative charges are arranged within an atom. This fundamental question has implications for how we understand chemical bonding, material properties, and even the behavior of biological molecules.
Atomic Structure: A Quick Review
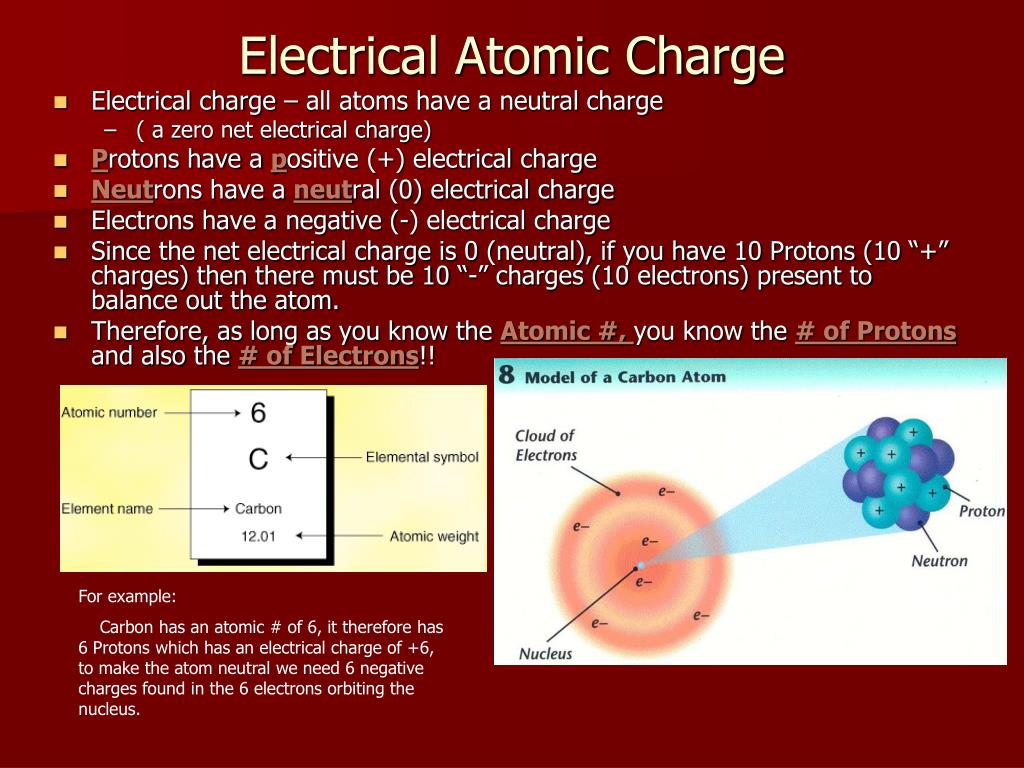
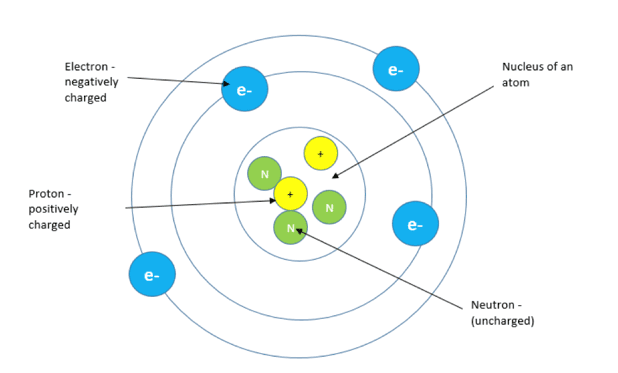
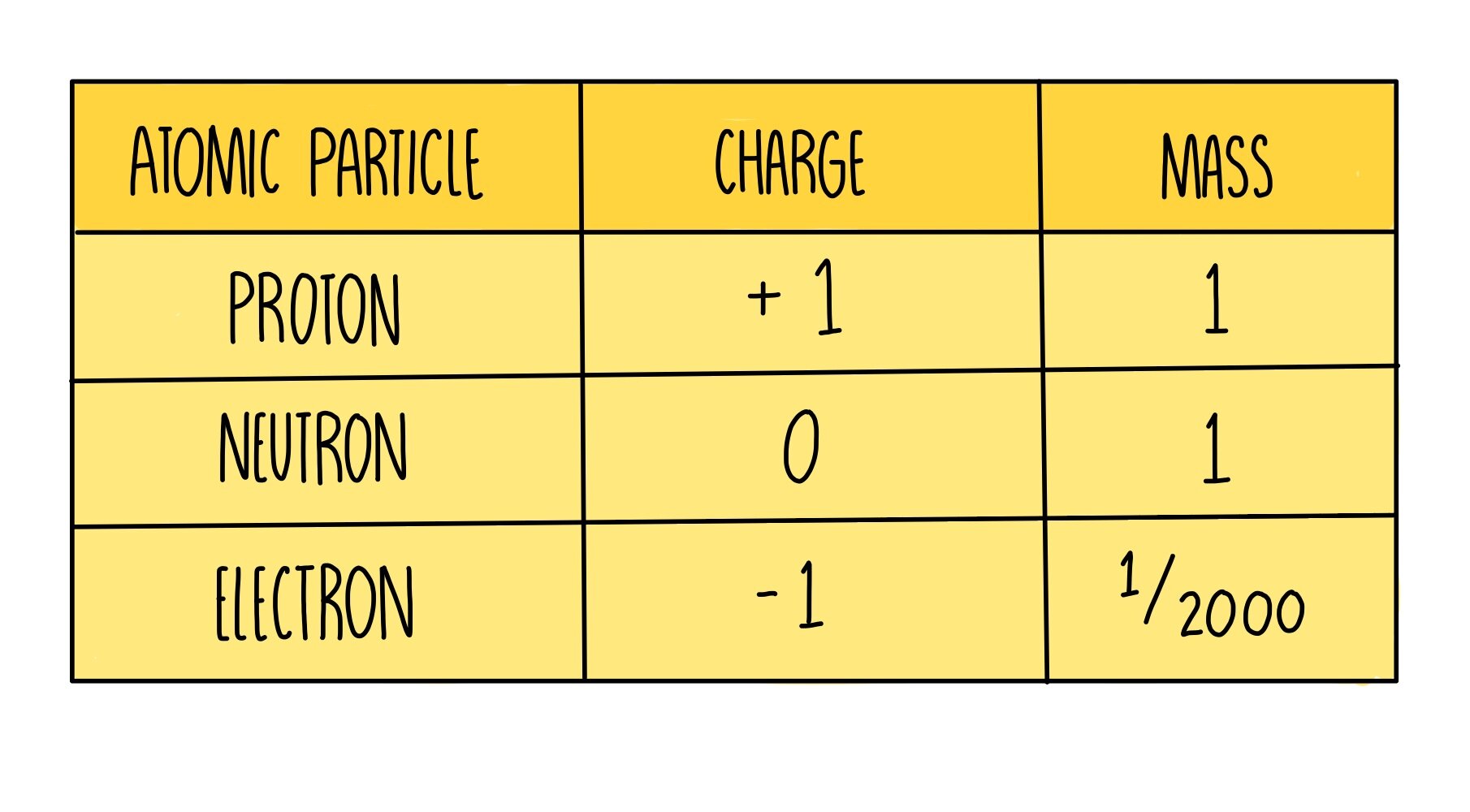
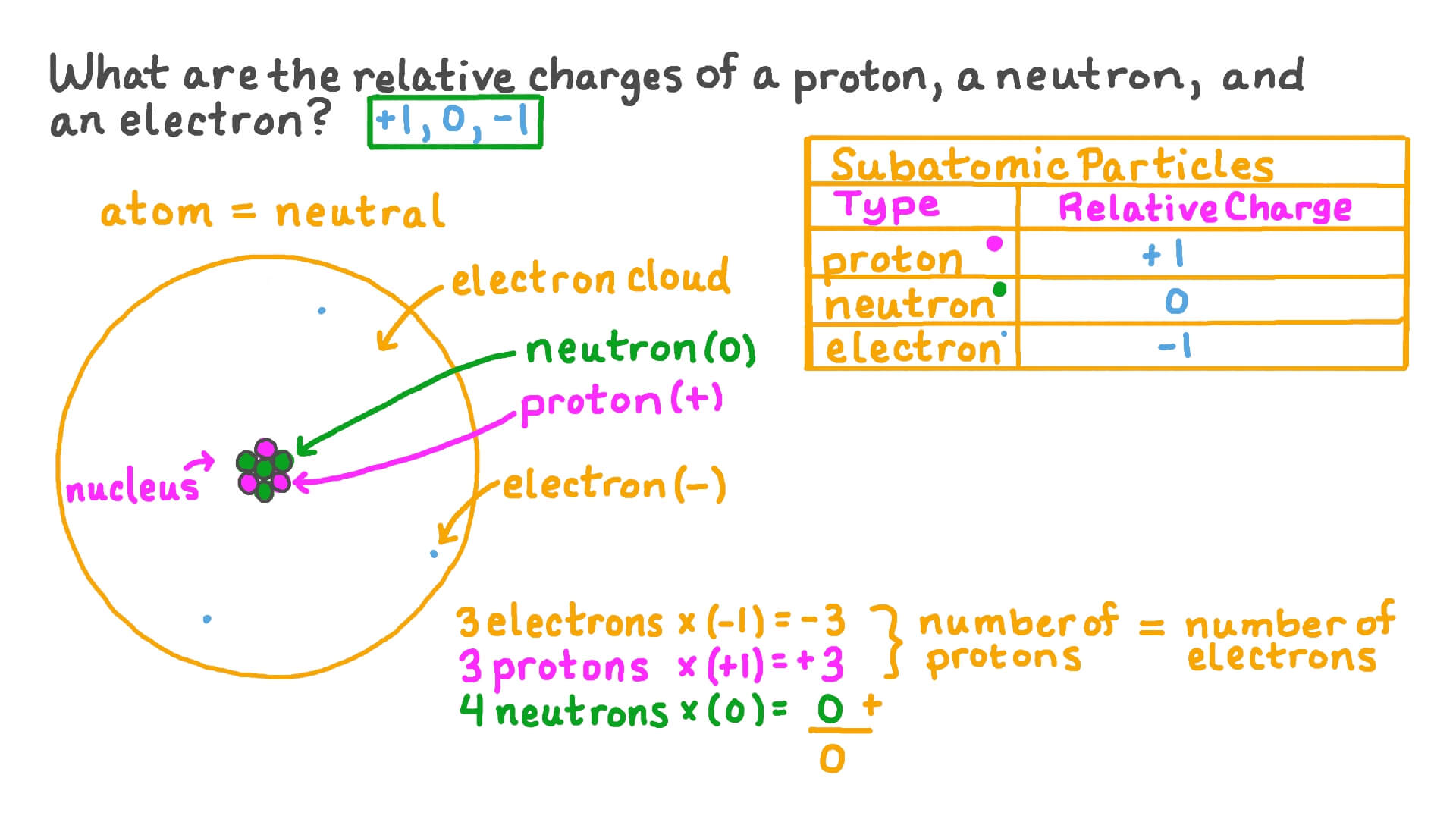
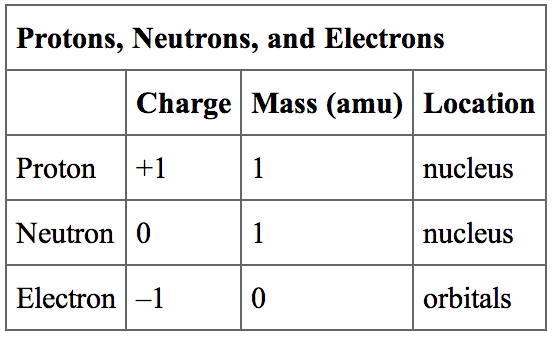
Atoms, the building blocks of matter, are composed of three primary subatomic particles: protons, neutrons, and electrons. Protons carry a positive charge, electrons a negative charge, and neutrons have no charge. The nucleus, located at the center of the atom, contains protons and neutrons, while electrons orbit the nucleus.
The question of charge distribution within an atom concerns how these particles are arranged. Specifically, how the positive charge of the protons is balanced by the negative charge of the electrons and how this arrangement affects the atom's behavior.
The Modern Atomic Model: Nuances in Charge Distribution
The currently accepted model, built upon the work of scientists like Ernest Rutherford and Niels Bohr, posits a dense, positively charged nucleus surrounded by negatively charged electrons in specific energy levels or orbitals. These orbitals aren't simple, fixed paths, but rather probability distributions describing where an electron is likely to be found at any given time.
One could argue the charge is distributed as follows: The positive charge is concentrated in the nucleus, a tiny region containing protons. The negative charge is dispersed throughout the space surrounding the nucleus, described by electron orbitals.
Beyond the Basics: Quantum Mechanics and Charge Density
However, this simplified picture becomes more complex when considering quantum mechanics. Quantum mechanics provides a more sophisticated view of electron behavior, describing electrons not as point-like particles but as waves.
Therefore, the charge distribution isn't static or uniform. It’s a dynamic and probabilistic phenomenon described by a charge density, a measure of the probability of finding an electron at a particular point in space.
Dr. Anya Sharma, a theoretical chemist at the National Institute of Standards and Technology (NIST), explains: "The charge distribution is not a uniform sphere, but a complex, three-dimensional shape dictated by the solutions to the Schrödinger equation for that atom. This shape dictates how the atom interacts with other atoms to form molecules."
Experimental Evidence and Observations
Numerous experimental techniques support the current understanding of charge distribution. X-ray diffraction, for example, allows scientists to map electron density in crystals, revealing the shapes of electron orbitals and confirming the non-uniform distribution of charge.
Spectroscopy provides information about the energy levels of electrons in atoms, supporting the idea that electrons occupy specific orbitals with distinct energy levels and charge distributions. These experiments solidify our understanding.
Implications and Societal Impact
Understanding charge distribution at the atomic level has profound implications across various fields. In chemistry, it allows us to predict how atoms will interact to form molecules, designing new materials with specific properties.
In medicine, it enables the development of new drugs that target specific molecules in the body. The entire field of nanotechnology also relies on the manipulation of atoms and molecules at the nanoscale, requiring a deep understanding of charge distribution.
Furthermore, advances in computing power have allowed scientists to perform increasingly accurate simulations of atomic charge distributions, further refining our understanding and enabling new discoveries.
"Accurate modeling of atomic charge is crucial for accurate simulations of materials," says Professor Kenji Tanaka, a computational materials scientist at the University of Tokyo.
Ultimately, the distribution of charge in an atom is best described as a concentration of positive charge in the nucleus, balanced by a dynamic, probabilistic distribution of negative charge described by electron orbitals. This understanding is not merely an abstract scientific concept but a cornerstone of modern science and technology, with wide-ranging implications for society.