Why Does Splitting An Atom Release Energy
The pursuit of harnessing the atom's power has shaped the 20th and 21st centuries, driving both unprecedented technological advancements and raising profound ethical questions. The very foundation of this power rests on a seemingly simple principle: splitting an atom releases energy. But understanding why this happens requires delving into the heart of nuclear physics, a realm where mass and energy are interchangeable, and the forces holding matter together are unimaginably strong.
At its core, the phenomenon of nuclear fission – the splitting of an atom – involves a delicate balance of forces and a subtle conversion of mass into energy. The process hinges on the structure of the atomic nucleus, the binding energy that holds it together, and Einstein's famous equation, E=mc². This article will explore these fundamental concepts, examine the different isotopes involved, and consider the implications for both nuclear power and nuclear weapons.
The Binding Energy of the Nucleus
An atom’s nucleus comprises protons and neutrons, collectively known as nucleons. These particles are bound together by the strong nuclear force, an incredibly powerful attraction that overcomes the electrostatic repulsion between the positively charged protons.
This force is incredibly strong at short distances, but rapidly weakens with increasing separation. The energy required to overcome the strong nuclear force and separate all the nucleons in a nucleus is known as the binding energy.
The higher the binding energy per nucleon, the more stable the nucleus. This concept is crucial to understanding why certain atoms undergo fission.
Mass Defect and Einstein's Equation
A fascinating aspect of nuclear physics is the concept of mass defect. When nucleons bind together to form a nucleus, the mass of the resulting nucleus is slightly less than the sum of the masses of the individual nucleons.
This missing mass is not lost; it is converted into energy, according to Einstein’s famous equation: E=mc², where E is energy, m is mass, and c is the speed of light. This equation demonstrates the equivalence of mass and energy; a small amount of mass can be converted into a tremendous amount of energy, and vice versa.
This energy is released during the formation of the nucleus and represents the binding energy that holds the nucleus together.
Nuclear Fission: Splitting the Atom
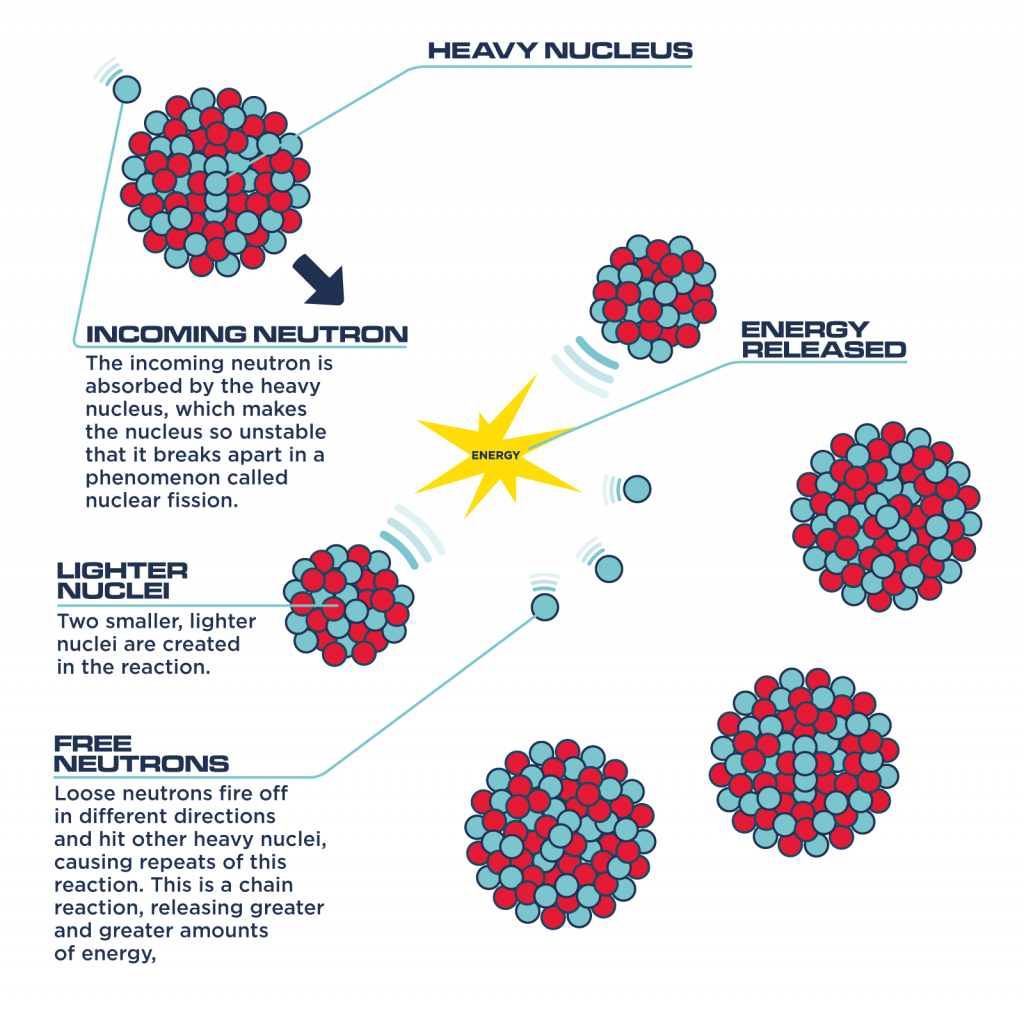
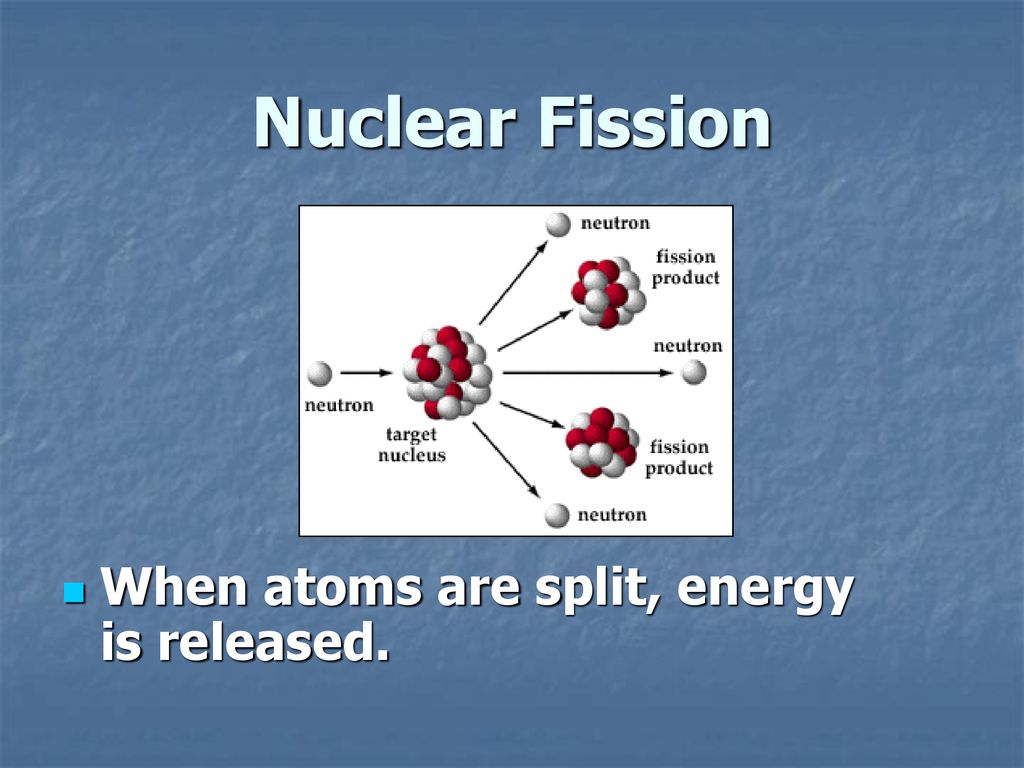
Fission typically occurs when a heavy nucleus, such as uranium-235 or plutonium-239, absorbs a neutron. This added neutron destabilizes the nucleus, causing it to deform and eventually split into two smaller nuclei, called fission fragments.
The combined mass of these fission fragments is less than the mass of the original nucleus plus the neutron that initiated the fission. This mass difference is converted into kinetic energy of the fission fragments and emitted neutrons, as well as gamma radiation, according to E=mc².
In essence, the fission process releases the binding energy that was holding the original nucleus together.
Isotopes and Chain Reactions
Not all isotopes are capable of sustaining a chain reaction. For instance, uranium exists in several isotopic forms, including uranium-238 (U-238) and uranium-235 (U-235). U-235 is fissile, meaning it can sustain a chain reaction, while U-238 is not.
When a U-235 nucleus undergoes fission, it releases not only energy but also several neutrons. These neutrons can then strike other U-235 nuclei, causing them to undergo fission as well, thereby creating a self-sustaining chain reaction.
The control of this chain reaction is critical in nuclear reactors. Control rods made of neutron-absorbing materials, such as boron or cadmium, are used to regulate the number of neutrons available to initiate further fission events, preventing a runaway reaction.
Implications and Applications
The discovery of nuclear fission has had profound implications for both energy production and weapons development. Nuclear power plants utilize controlled nuclear fission to generate electricity.
The heat produced from the fission of uranium fuel is used to boil water, creating steam that drives turbines connected to generators. This process offers a carbon-free alternative to fossil fuel-based power generation, but also carries the risks associated with nuclear waste disposal and the potential for accidents like Chernobyl or Fukushima.
Conversely, uncontrolled nuclear fission is the basis of nuclear weapons. In these weapons, a critical mass of fissile material is rapidly assembled, leading to an exponential increase in the rate of fission and a massive release of energy in a short period.
Challenges and Future Directions
While nuclear fission offers a potentially abundant source of energy, it also presents several challenges. The safe disposal of radioactive waste remains a significant concern, as some isotopes have half-lives of thousands of years.
Research is ongoing to develop advanced reactor designs that are inherently safer and produce less waste. These include breeder reactors, which can convert non-fissile isotopes into fissile ones, and fusion reactors, which aim to harness the energy released when light nuclei fuse together.
Another avenue of research focuses on transmutation, a process that involves converting long-lived radioactive isotopes into shorter-lived or stable ones.
In conclusion, the release of energy during nuclear fission is a direct consequence of the mass defect and the conversion of mass into energy according to Einstein’s E=mc². It's a testament to the immense forces at play within the atomic nucleus and the potential energy stored within. As we continue to grapple with the challenges of energy security and climate change, a deeper understanding of nuclear physics and responsible innovation in nuclear technologies will be crucial. The ongoing pursuit of fusion energy, in particular, holds the promise of a cleaner, safer, and virtually inexhaustible energy source for future generations, but much research and development remains before that promise can be realized.













+–+Nuclear+fission.jpg)

