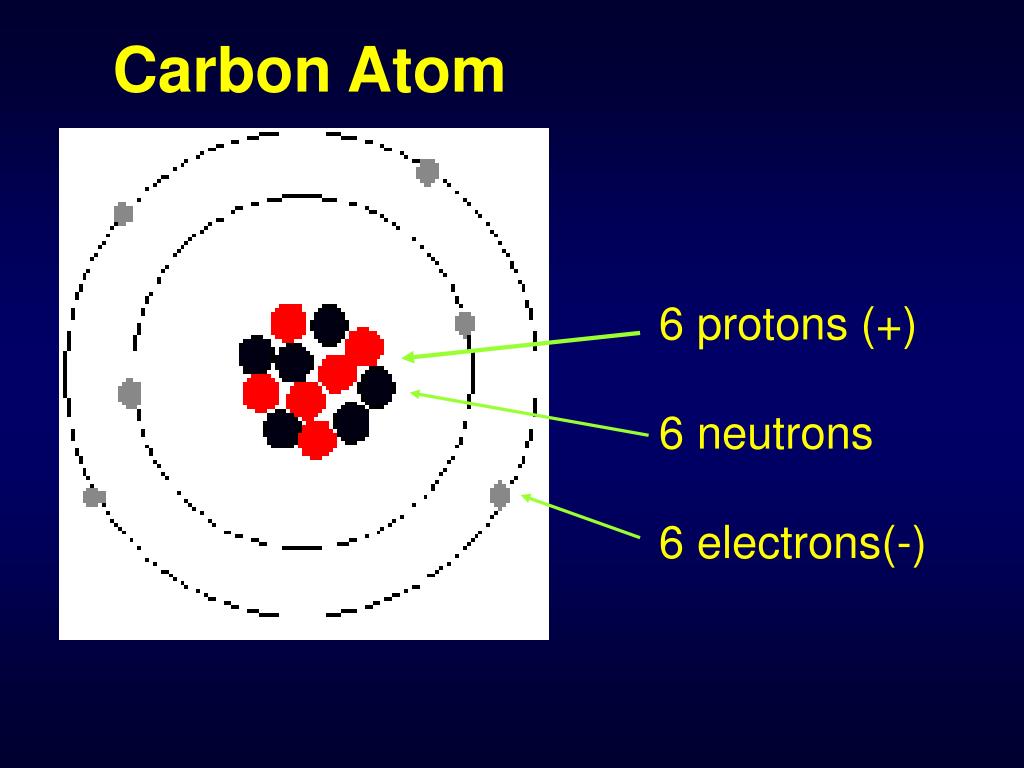
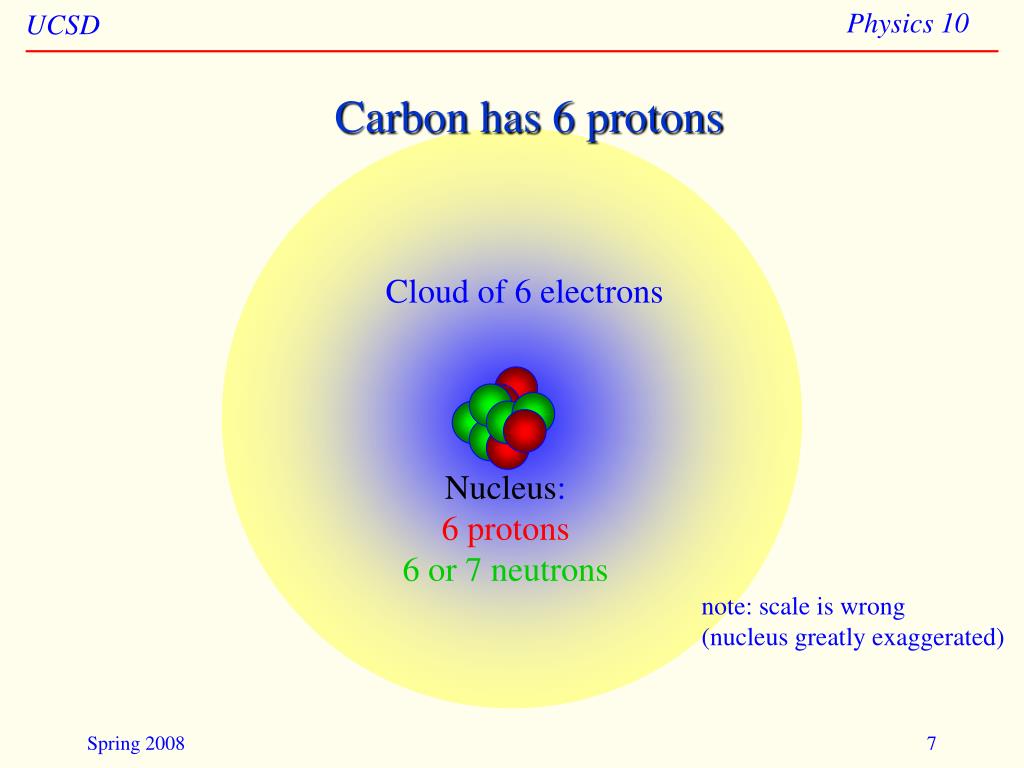
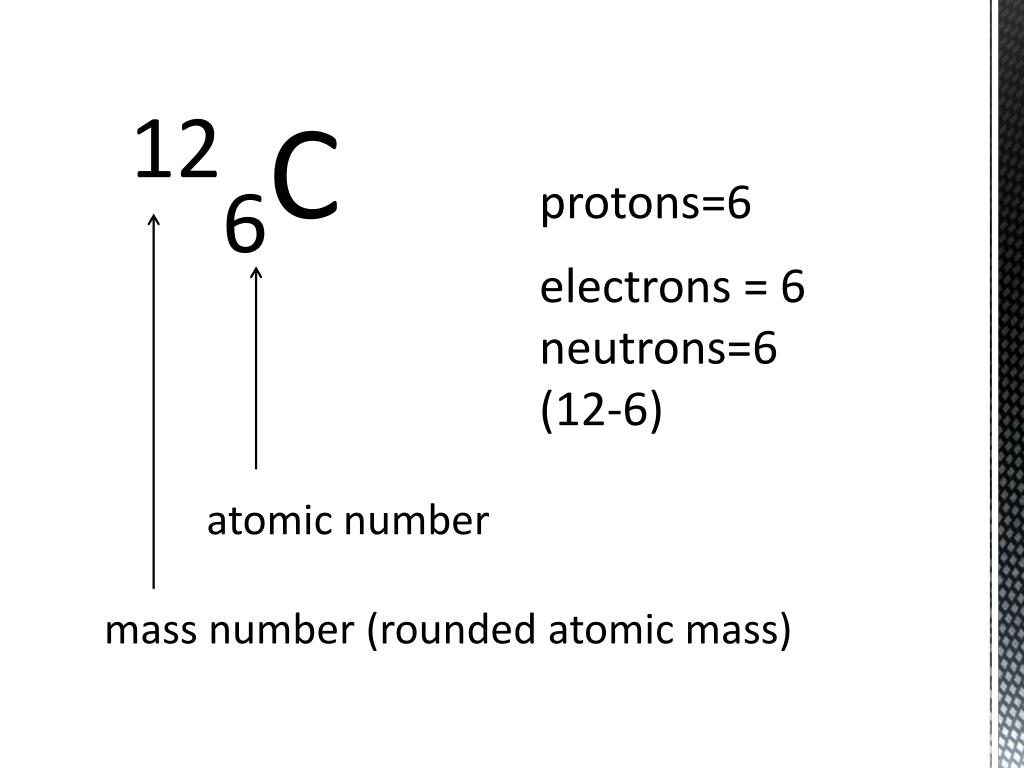
6 Protons 6 Neutrons 6 Electrons Mass Number
Ever looked at a piece of charcoal and thought, "Wow, that's secretly a mathematical marvel?" Probably not! But beneath its sooty exterior lies a story of tiny particles, surprisingly neat numbers, and the foundation of… well, everything!
The Carbon Crew: A Party of 18!
Let's talk about carbon. It's the backbone of life, the star of diamonds, and the main ingredient in, yes, charcoal. But what *is* carbon, really? It all comes down to a party of 18, dancing within each carbon atom.
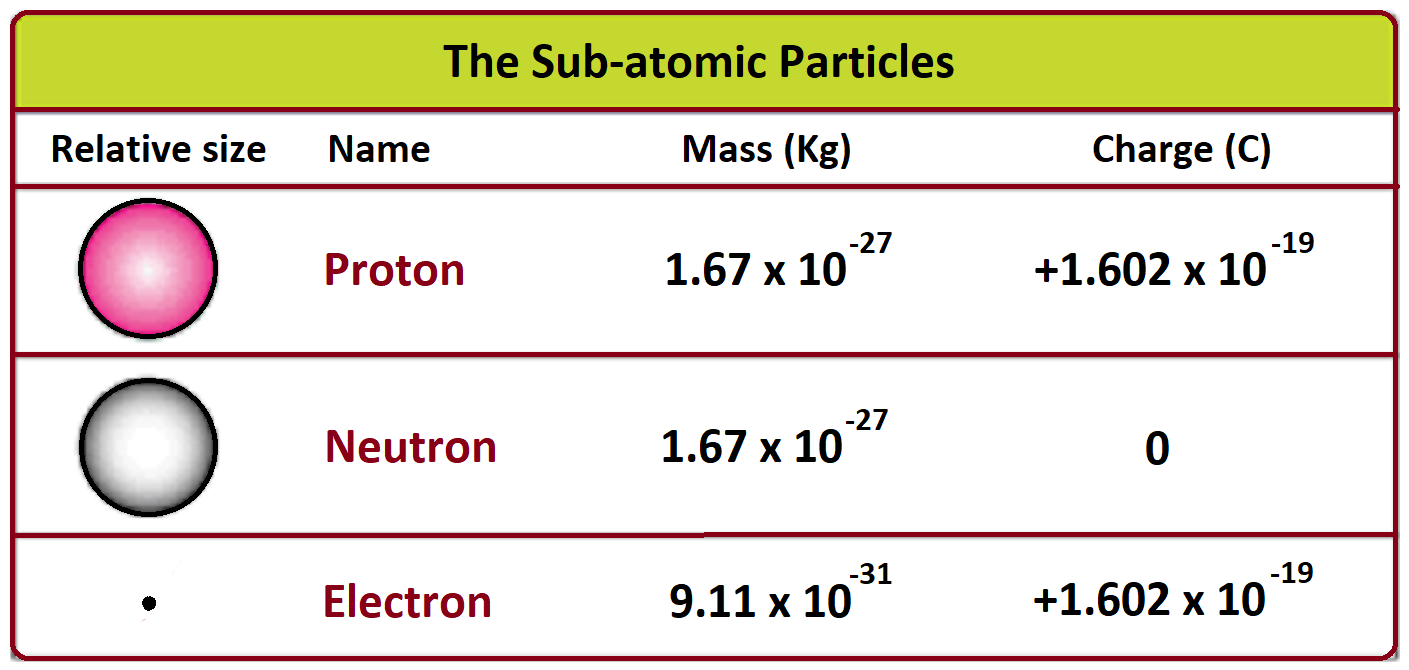
Imagine these 18 partygoers are broken down into three types: 6 Protons, 6 Neutrons, and 6 Electrons. Each plays a crucial role in defining what makes carbon carbon.
Protons: The Identity Checkers
Think of protons as the bouncers at the club. They're positively charged, and they reside in the nucleus, the atom's VIP section. The number of protons *defines* the element.
Carbon *always* has 6 protons. Change the number of protons, and suddenly, you're not carbon anymore! You might be oxygen, or even nitrogen! It's a pretty strict entry policy.
Neutrons: The Neutral Buddies
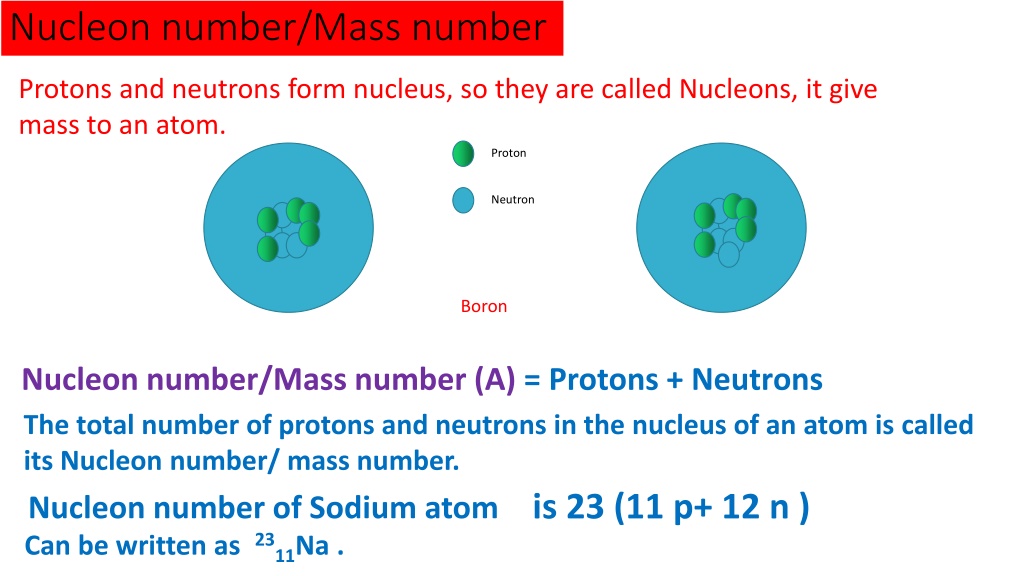
Neutrons, also chilling in the nucleus, are the neutral ones. They don't have a charge, but they do contribute to the atom's overall weight. They are like the solid support to the proton bouncers.
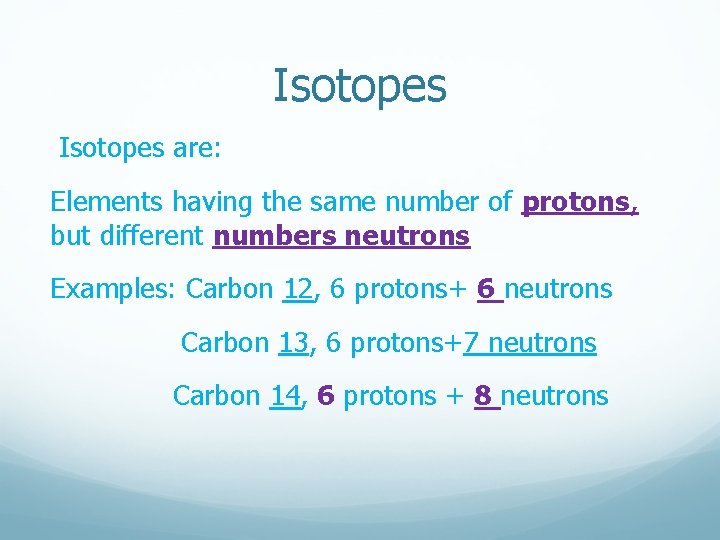
Carbon usually has 6 neutrons, but sometimes it has 7 or 8. This creates different versions of carbon called isotopes. It's like having siblings with slightly different builds, but still clearly part of the family!
Electrons: The Energetic Dancers
Spinning around the nucleus in a wild frenzy are the electrons. They're negatively charged and incredibly light. Imagine them as the dancers, constantly moving and grooving.
In a neutral carbon atom, there are always 6 electrons. The number of electrons determines how carbon interacts with other atoms – how it forms bonds and creates molecules.
Mass Number: The Total Headcount
So, we've got 6 protons, 6 neutrons, and 6 electrons. But how do we quantify the overall “size” of the atom? That’s where the mass number comes in!
The mass number is simply the total number of protons and neutrons in the nucleus. Why only protons and neutrons? Because electrons are so light; they barely contribute to the atom's weight.
For most carbon atoms, the mass number is 12 (6 protons + 6 neutrons). But for that carbon sibling with 7 neutrons, the mass number is 13. We call it Carbon-13! For that carbon sibling with 8 neutrons, the mass number is 14. We call it Carbon-14!
“The mass number is like the bouncer taking a quick headcount of the important guests in the VIP section.”
Why Does This Matter?
Okay, so we've counted particles. But why should you care? Because these tiny numbers dictate everything from the hardness of a diamond to the way your body functions. Carbon-14, the isotope with 8 neutrons, is used in carbon dating, which allows scientists to determine the age of ancient artifacts.
Without understanding these fundamental building blocks, we wouldn't have modern medicine, advanced materials, or even a decent understanding of the universe! Next time you see a diamond or light a charcoal grill, remember the dance of the 18 – the protons, neutrons, and electrons – and the secret mathematical marvel that makes it all possible.
It's a tiny world with huge implications, all starting with the simple numbers: 6, 6, and 6. A surprisingly powerful party of particles!

..jpg)


+%26+electrons+(6)+because+their+atomic+number+is+the+same+(6)+-+They+each+have+different+amounts+of+neutrons+so+their+mass+numbers+are+different+-+carbon-12+%3D+6+neutrons+(12-6)+-+carbon-13+%3D+7+neutrons+(13-6)+-+carbon-14+%3D+8+neutrons+(14-6).jpg)





+has+the+atomic+number+of+6%2C+how+many+protons+does+it+have+____________.+How+many+electrons+____________..jpg)


