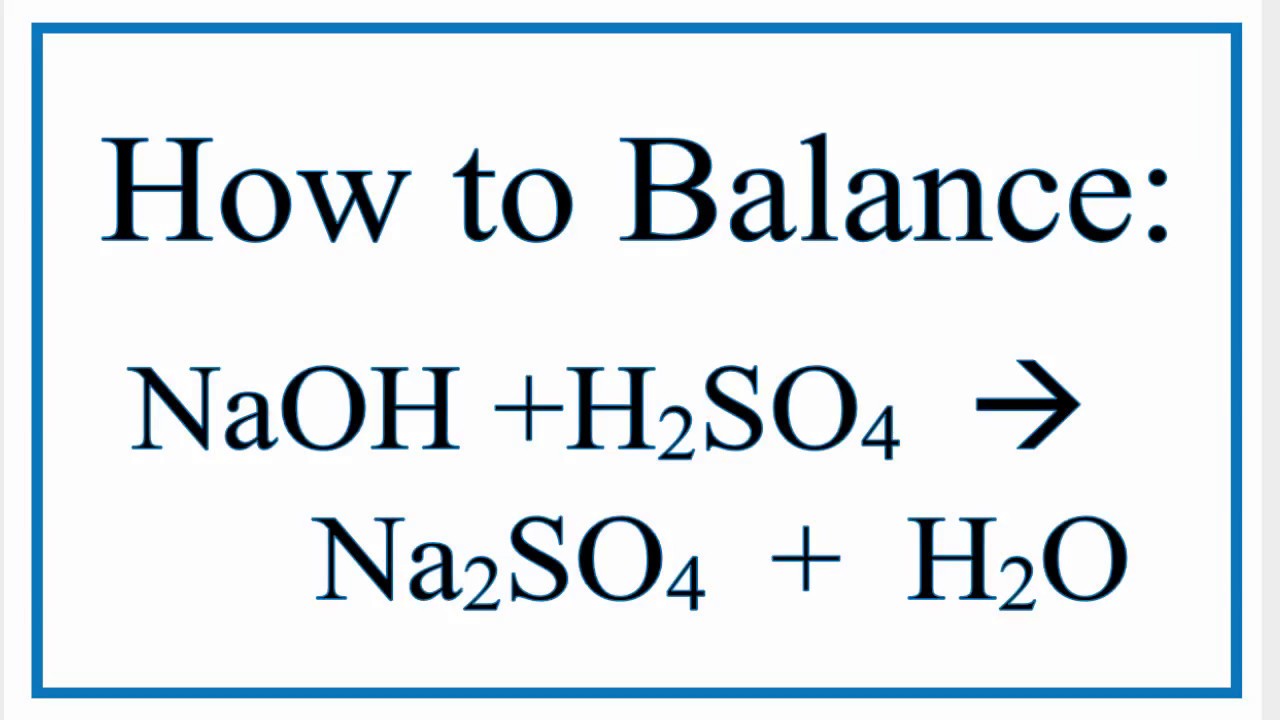
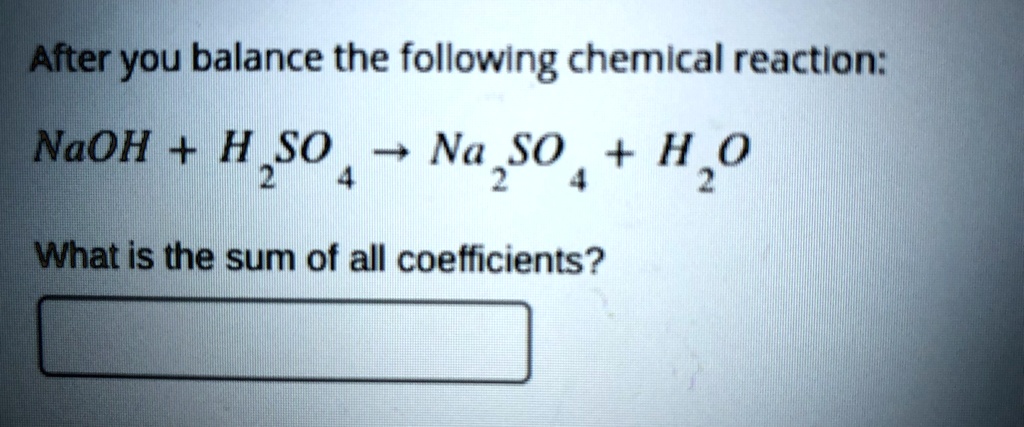
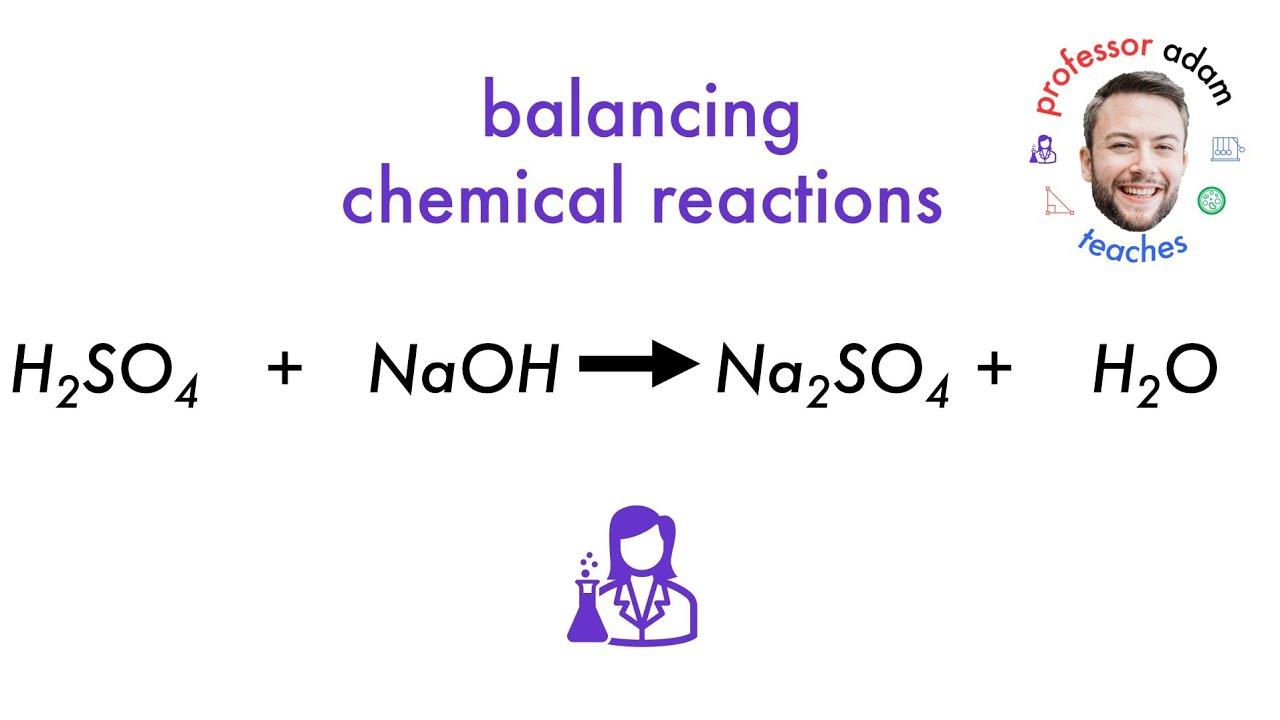
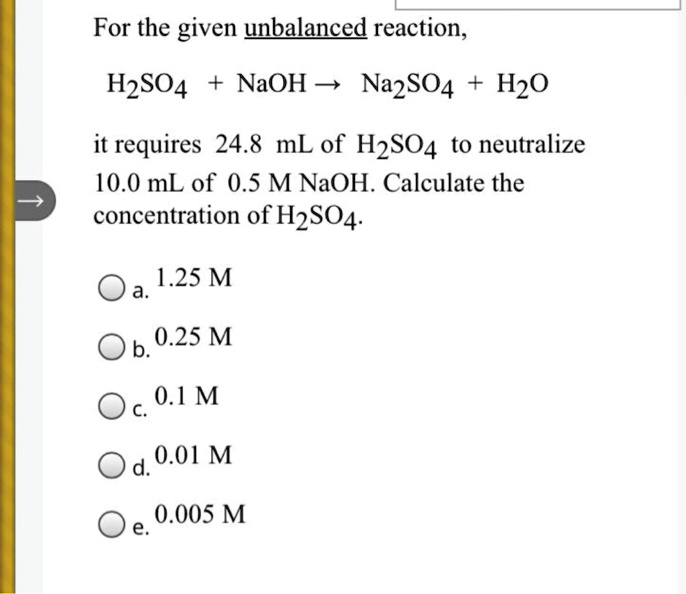
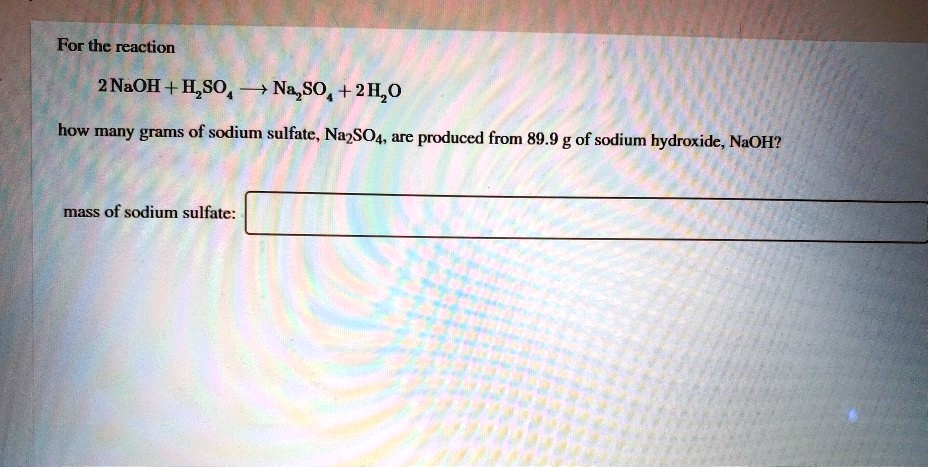
Naoh H2so4 Na2so4 H2o Type Of Reaction

Chemistry! It's not just bubbling beakers and mysterious smells. Sometimes, it's like watching a really good magic trick. Except, instead of pulling rabbits out of hats, we're rearranging tiny building blocks called atoms.
Let's talk about a chemical story with some interesting characters. We've got NaOH (sodium hydroxide), H2SO4 (sulfuric acid), Na2SO4 (sodium sulfate), and good old H2O (water).
The Players in Our Chemical Drama
Think of NaOH as a strong, opinionated character. It's a base, which means it loves to react with acids. Imagine it as the superhero ready to neutralize any villainous acid!
Then there’s H2SO4, a powerful acid. It's a bit like the grumpy boss that needs to be calmed down. You wouldn't want to mess with it unless you know what you're doing.
And Na2SO4? That's the result of their interaction, a salt! It's the calm, collected product of a job well done. Salts are pretty stable.
Lastly, we have H2O, water. It's like the ever-present audience, sometimes a participant, but always essential. Water helps everything happen.
The Big Reaction: A Chemical Dance
Now, imagine NaOH and H2SO4 meeting. It's not a quiet chat. It’s a full-blown reaction! They combine in a way that releases energy, like a little explosion of activity at a microscopic level.
Think of it as a chemical tango. The acid and base neutralize each other in a heated, passionate dance. When the music stops, they've transformed into something new.
The product of this chemical tango? Na2SO4 and H2O. This is a classic example of neutralization.
Why is This So Cool?
This reaction is cool because it's fundamental. It shows how acids and bases interact, cancelling each other out in a process. It's like balancing the scales.
It’s also predictable. When you mix these two chemicals, you know what to expect. It’s not always that way with chemistry! This predictability is kind of comforting.
Plus, it's used everywhere! From making soaps to cleaning products, this type of reaction is crucial. Chemistry plays a huge role in our daily lives.
Beyond the Basics
Now, chemists get really excited about the details. They measure the heat released (exothermic reaction!), and calculate the precise amounts of each chemical needed. But you don't need to be a chemist to appreciate it.
They also worry about things like concentration and stoichiometry, which are fancy words for figuring out how much of each ingredient is needed. That’s like baking but with atoms.
You can explore this reaction further. Search for videos of acid-base neutralizations, or maybe find a safe, supervised experiment to try.
So, What's the Reaction Type?
This whole scenario falls under the category of a neutralization reaction. Acids and bases react to neutralize each other. Hence, the name.
And because this specific reaction results in a salt (Na2SO4) and water (H2O), it's also a double displacement reaction. Think of it as a swap-and-switch.
It's a win-win for everyone involved. Well, maybe not the acid and base themselves, but certainly for the resulting products!
"Chemistry is necessarily an experimental science: its conclusions are drawn from data, and its principles supported by evidence from facts." - Michael Faraday
Dive Deeper!
Don't be intimidated by the chemical formulas! Break them down and focus on the interactions.
Chemistry is just a language, and with a little practice, you can learn to speak it. Each element is like a word and reaction is like sentences. Everything is a story.
The world around us is full of chemical reactions, waiting to be discovered. So go forth and explore the chemistry of everything!