The 2400 Year Search For The Atom

Imagine searching for something you can't even see, something so ridiculously tiny that a million of them could line up on the head of a pin. Sounds like a fool's errand, right? Well, brave souls have been doing exactly that for over 2400 years, and their quest is the hunt for the atom!
Our story begins way back in ancient Greece, around 440 BC. Two thinkers, Leucippus and his student Democritus, had a wild idea. What if you kept cutting something in half, again and again?
They figured there had to be a point where you couldn't cut it anymore, an indivisible particle. They called these uncuttable bits atomos, meaning "uncuttable." Basically, they were the original atom hunters!
Now, these guys didn’t have fancy microscopes or laboratories. They did a lot of thinking and arguing, which, let's be honest, is a pretty fun way to spend an afternoon. They were onto something big, even if they couldn’t *prove* it with, you know, *evidence*.
Fast forward a couple of millennia, and the scientific method finally came into style. Instead of just pondering, people started experimenting. Along came John Dalton in the early 1800s, who essentially dusted off Democritus's idea and gave it a scientific makeover.
Dalton proposed that all matter is made of atoms. He also declared that all atoms of a given element are identical. Suddenly, the atom was back in business!
Then things got *really* interesting. Imagine you finally find that thing you've been searching for, only to discover it's made of even smaller things! That's basically what happened with the atom.
J.J. Thomson, in 1897, discovered the electron, a tiny negatively charged particle. This discovery proved that atoms weren't actually indivisible. The atom was, dare we say, smashable!
Thomson came up with the "plum pudding model" of the atom, where electrons were scattered like plums in a positively charged pudding. It was a tasty analogy, but soon to be proven wrong.
The Nuclear Family
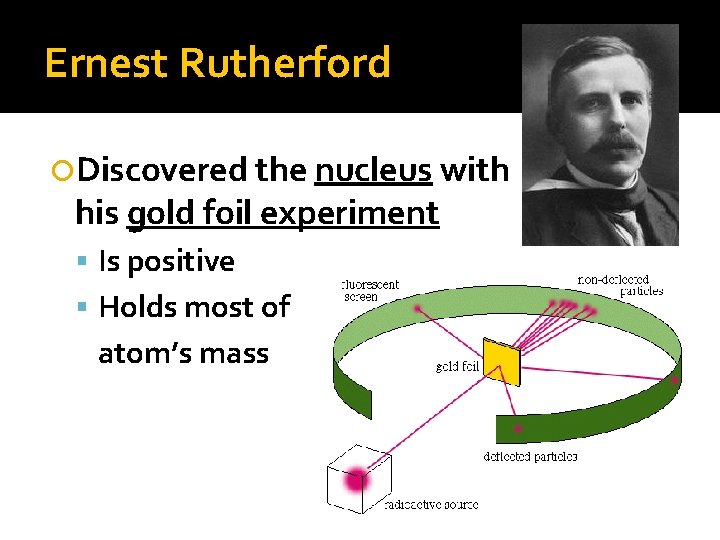
Ernest Rutherford came along with his famous gold foil experiment. He shot tiny particles at a thin sheet of gold foil and observed how they scattered. The results blew everyone's minds.
Most particles passed straight through, but some bounced back as if they hit something solid! This led Rutherford to propose the existence of a tiny, dense, positively charged nucleus at the center of the atom.
Think of it like this: If the atom were a football stadium, the nucleus would be a marble on the 50-yard line. The rest is mostly empty space, occupied by whizzing electrons. Crazy, right?
Even Smaller Stuff
But the atom story doesn’t end there! Scientists later discovered that the nucleus itself is made of even smaller particles: protons (positive charge) and neutrons (no charge).
So, the atom isn’t indivisible, and protons and neutrons are (mostly) the building blocks. For the average person, this is the level of understanding that is taught in school.
And even those protons and neutrons? Yep, they're made of even *smaller* particles called quarks. These are held together by gluons. It's atoms all the way down!
This 2400-year hunt has involved countless scientists, mind-boggling experiments, and a whole lot of really big ideas about something incredibly small. It demonstrates human curiousity and persistence.
So, next time you're staring at your phone, remember that everything you see is made of atoms. And those atoms are made of even smaller things. The search continues!
It also reminds us that even if we can't see something, it doesn't mean it's not there. Keep exploring, keep questioning, and who knows? Maybe *you'll* be the one to make the next big atomic discovery!












![The 2400 Year Search For The Atom [CHEMISTRY TEACHING/LEARNING AIDS] Discovery of the Atom, History of](https://images.squarespace-cdn.com/content/v1/5f02d28f35d64d2a5022eeb1/1598681992876-Y4TGY22GJHNPDCYCJFC4/atomic_chemistry.png)