The Determination Of Keq For Fescn2+ Lab Answers
Ever wondered how scientists figure out how much a reaction really likes to happen? I'm talking about the magical world of chemical equilibrium! Think of it like a party where some people are dancing (reactants) and others are chilling on the couch (products).
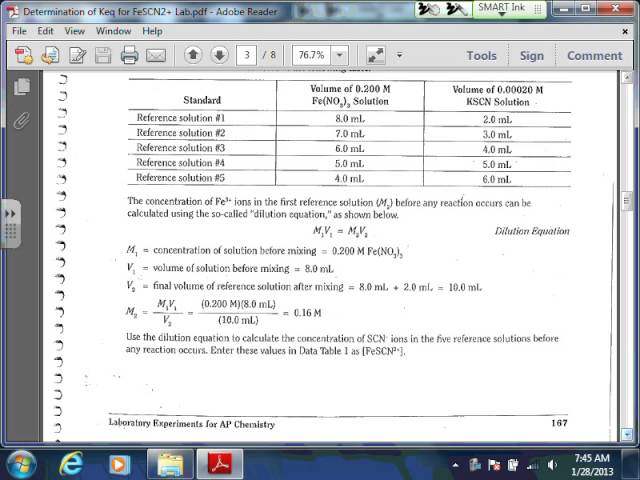
It is a determination of Keq for FeSCN2+. Finding the Keq value is like figuring out the ultimate popularity contest at that party.
This lab's all about getting hands-on. No more boring textbook stuff! We get to mix stuff, see colors change, and use fancy instruments to measure how much of everything's hanging around at the equilibrium.
First, there is a mixing solutions part. You're probably thinking, "Mixing? Seriously?" But trust me, it's like being a mad scientist for an afternoon! Okay, maybe a *slightly* less mad scientist.
We are using solutions with iron and thiocyanate. It’s important to be precise while doing the mixing. Every drop counts to getting the right results!
Color Me Amazed!
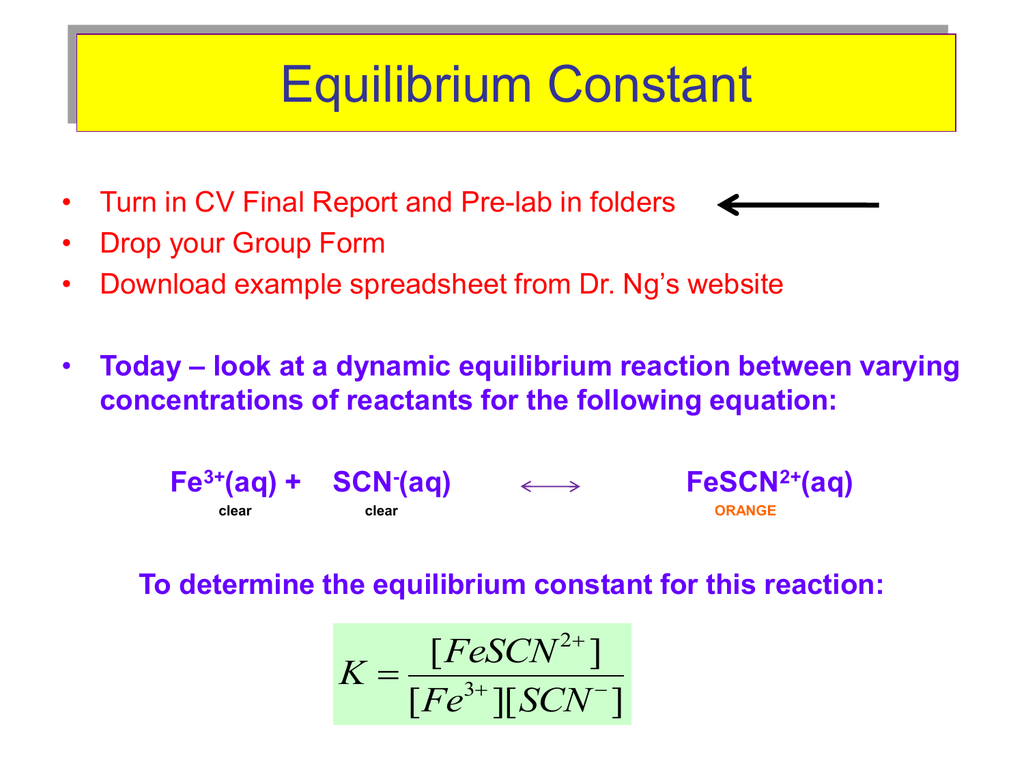
Here's where things get visually exciting! When iron ions (Fe3+) meet thiocyanate ions (SCN-), something beautiful happens. They form FeSCN2+, which has a vibrant, reddish-orange color. It's like a chemical sunset in a test tube!
The deeper the color, the more FeSCN2+ we've got. This color change is the key to unlocking the Keq value. We’re tracking the color intensity.
Think of it as reading the party atmosphere. The color tells us all about the balance between the "dancing" reactants and the "chilling" product.
The Spectrophotometer: Our Magical Color Reader
This lab involves a spectrophotometer. This amazing device shines light through your solution and measures how much light gets absorbed. It's like a high-tech vampire, but instead of blood, it craves light!
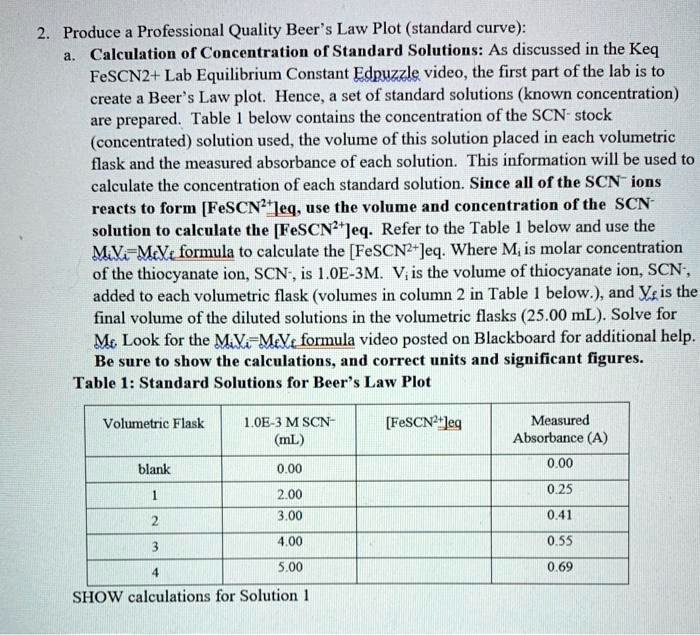
The amount of light absorbed is directly related to the concentration of FeSCN2+. The spectrophotometer helps us put a number on the color.
Essentially, we are using this tool to quantify just how much "party" is happening based on the color intensity. It gives us the data needed to calculate Keq.
The Thrill of the Calculation
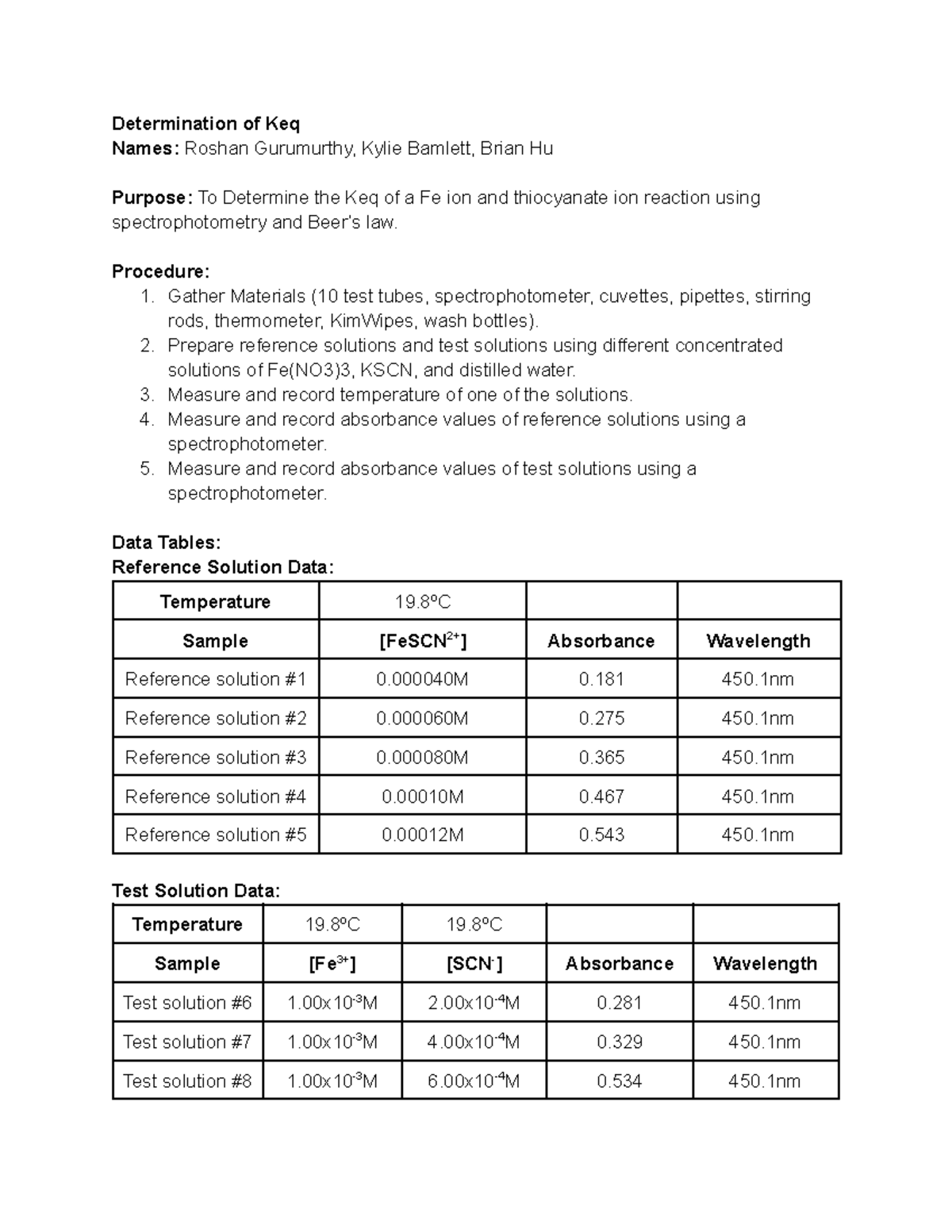
Now comes the part where we put on our thinking caps! Using the data from the spectrophotometer, we do some calculations to find the equilibrium concentrations of everything involved.
Then, we plug those values into the equilibrium expression to find Keq. Boom! We have it! Keq is the ratio of products to reactants at equilibrium.
This number tells us whether the reaction strongly favors the formation of FeSCN2+, weakly favors it, or sits somewhere in the middle. It's like the final score in the popularity contest.
"Finding Keq feels like solving a chemical mystery."
Why This Matters (And Why It's Fun!)
Understanding equilibrium is crucial in chemistry. It helps us predict how reactions will behave under different conditions. Think of it like predicting the future of the chemical world!
This experiment helps us explore how different factors can shift the balance of a reaction. What happens if you add more iron? What if you change the temperature? You get to find out! It's all about tinkering and seeing what happens.
Plus, there's something genuinely satisfying about seeing a concept come to life in the lab. This is not just some abstract number; it's a real-world measurement you obtained yourself!
Ready to Dive In?
The determination of Keq for FeSCN2+ might sound intimidating, but it is super interesting. It is all about mixing, observing colors, and using fancy tools to measure the state of a reaction.
So, the next time you hear about chemical equilibrium, remember the vibrant colors. Remember the thrill of discovery. And remember that you, too, can be a chemical detective, solving the mysteries of the molecular world!
You might even find yourself enjoying chemistry class a little more!





![The Determination Of Keq For Fescn2+ Lab Answers The Determination of Keq for [FeSCN2+] - ppt download](https://slideplayer.com/slide/14624657/90/images/5/Data+Table+3-+Results:+From+Calibration+Curve+(look+at+absorbance+and.jpg)



![The Determination Of Keq For Fescn2+ Lab Answers The Determination of Keq for [FeSCN2+] - ppt download](https://slideplayer.com/slide/14624657/90/images/1/The+Determination+of+Keq+for+[FeSCN2%2B].jpg)



