What Atom Has 6 Protons 6 Neutrons And 6 Electrons
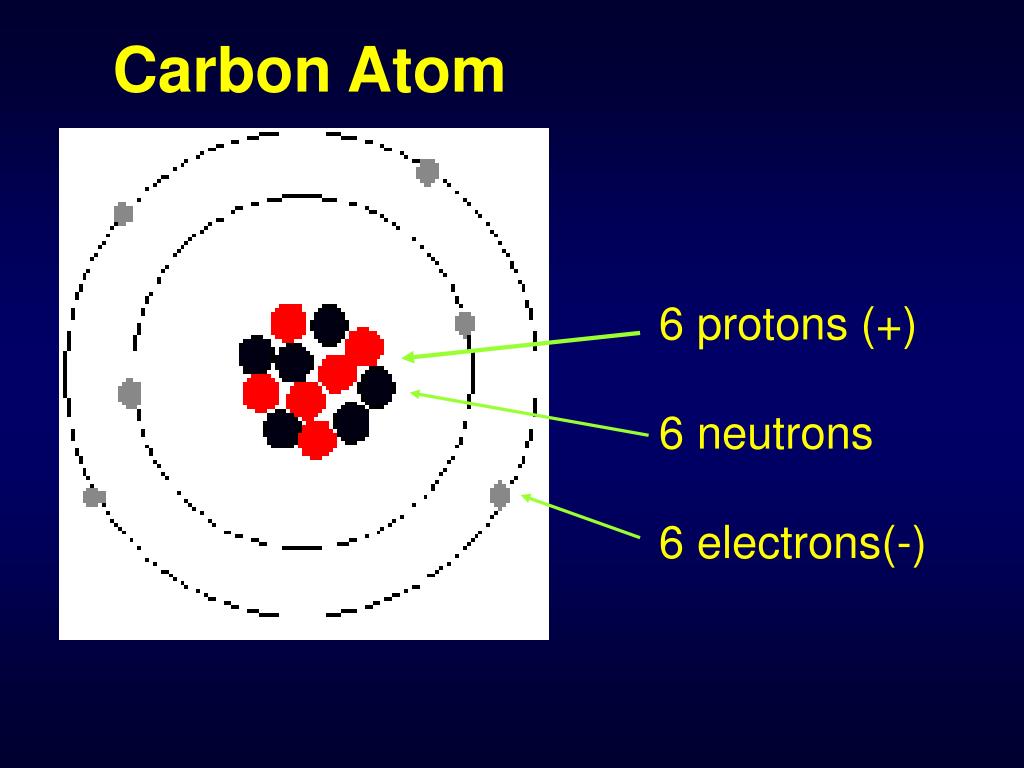
Ever wonder about the secret ingredient that makes life… well, life? It's not some mystical unicorn tear or the philosopher's stone. It's something far more common, yet equally magical: an atom with 6 protons, 6 neutrons, and 6 electrons.
Meet Our Star: Carbon!
That's right, we're talking about carbon! Don't let its simplicity fool you. This little atom is the backbone of pretty much everything you see (and are!), that's alive.
Think of it as the ultimate LEGO brick. It connects to other atoms to form incredible structures, like the DNA that makes you, well, *you*.
Why 6 of Everything? It's All About Balance.
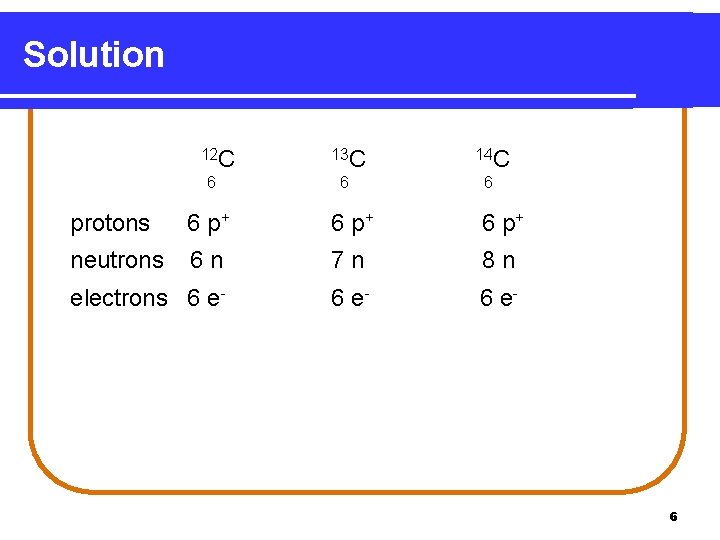
Protons are the positively charged particles, and their number defines what element an atom is. Six protons *always* means carbon.
The neutrons, hanging out in the nucleus with the protons, are neutral (hence the name!). Electrons are the negatively charged particles zipping around the nucleus. When the number of electrons equal the number of protons, the atom is neutral.
So, 6 protons, 6 neutrons, and 6 electrons create a perfectly balanced carbon atom, ready to build the world!
Carbon's Quirky Personality: Versatile and Social.
Carbon's superpower is its ability to form strong bonds with other atoms, especially itself! This “self-bonding” ability is a big reason why it can create such complex and diverse molecules.
It’s like the ultimate social butterfly, always ready to link up and create new relationships. This is known as covalent bonding.
This allows it to form long chains, rings, and even complex three-dimensional structures. Making it perfect to build big molecules!
From Diamonds to Graphite: A Carbon Family Reunion
Did you know that both diamonds and graphite (the stuff in your pencil) are made of *only* carbon? The difference lies in how those carbon atoms are arranged.
In diamonds, the atoms are tightly bonded in a strong, rigid structure. This is what makes them so hard and sparkly.
In graphite, the atoms are arranged in layers that can easily slide past each other. That’s why your pencil leaves a mark on the paper!
Talk about a family with wildly different personalities!
Carbon's Role in the Grand Scheme
But carbon's significance goes far beyond pretty gems and writing tools. It's absolutely essential for life as we know it!
Carbon is the key element in all organic molecules: carbohydrates, fats, proteins, and nucleic acids (like DNA and RNA).
These molecules are the building blocks of all living things on Earth. From the tiniest bacteria to the largest whales.
A Humble Atom, A Mighty Impact
So next time you admire a diamond ring, write with a pencil, or even just breathe, remember the incredible little atom with 6 protons, 6 neutrons, and 6 electrons: carbon.
It might be small, but its impact on the universe is truly gigantic. It's the silent architect of life, and that’s something to celebrate!
"We are stardust brought to life, then empowered by the cosmos to figure itself out." - Neil deGrasse Tyson
Think about that for a second!
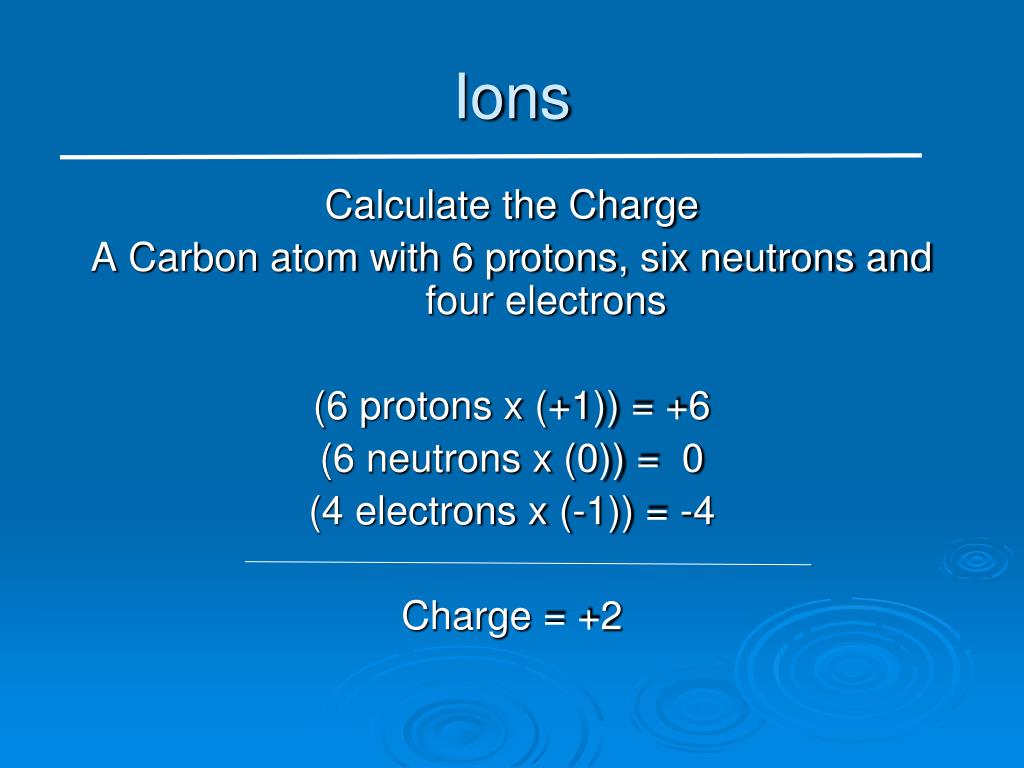


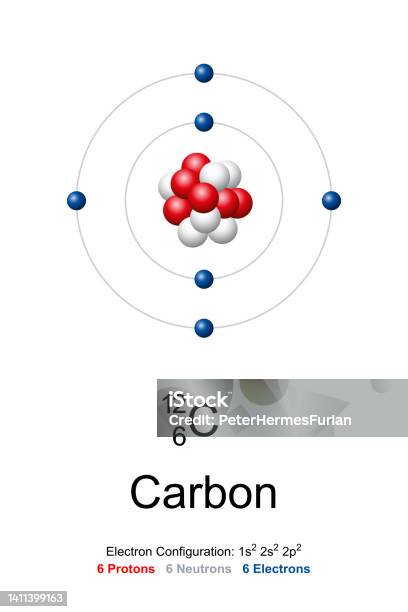

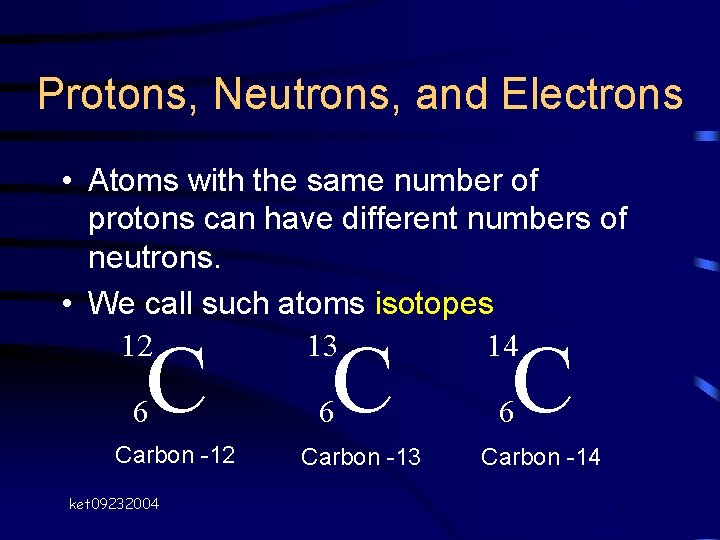









+%26+electrons+(6)+because+their+atomic+number+is+the+same+(6)+-+They+each+have+different+amounts+of+neutrons+so+their+mass+numbers+are+different+-+carbon-12+%3D+6+neutrons+(12-6)+-+carbon-13+%3D+7+neutrons+(13-6)+-+carbon-14+%3D+8+neutrons+(14-6).jpg)

