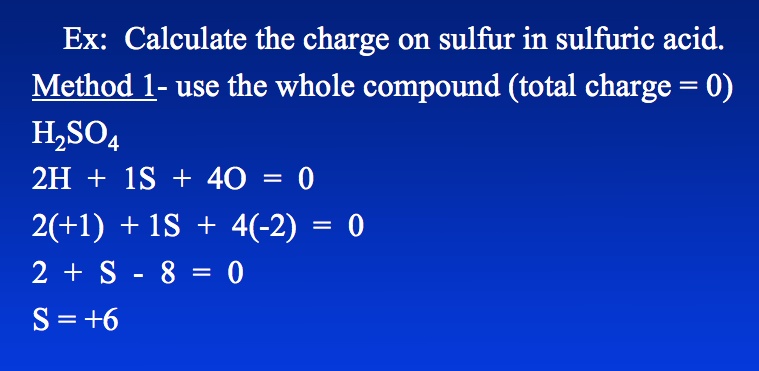What Is The Oxidation Number Of Sulfur In Sulfuric Acid
Ever wondered what secrets are hiding within the seemingly simple formula of sulfuric acid, H2SO4? Specifically, let's peek at what the oxidation number of our friend sulfur is. It's like uncovering a hidden superpower!
Don't worry, this isn't going to be a dreaded chemistry lecture. We'll think of oxidation numbers as charges atoms *pretend* to have when they're all playing nicely together in a compound.
Sulfur's Secret Life
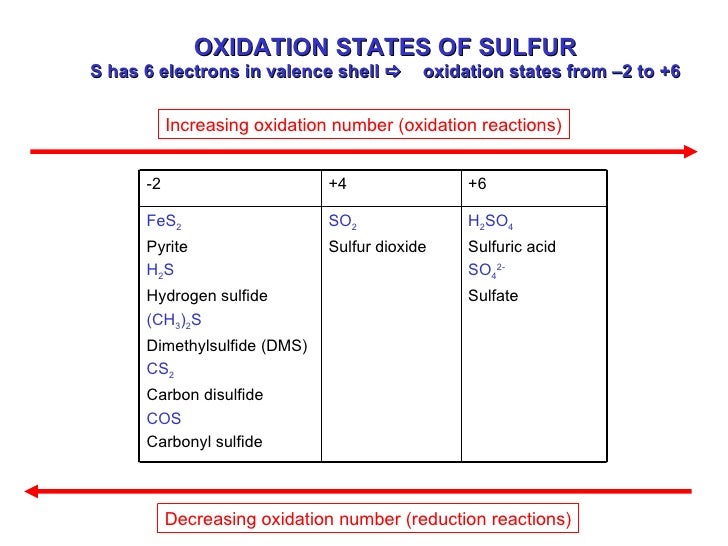
Imagine sulfur as a bit of a chameleon. It's versatile and can adopt different "personalities" depending on who it's hanging out with. That's because its oxidation number can change.
The Sulfuric Acid Scene
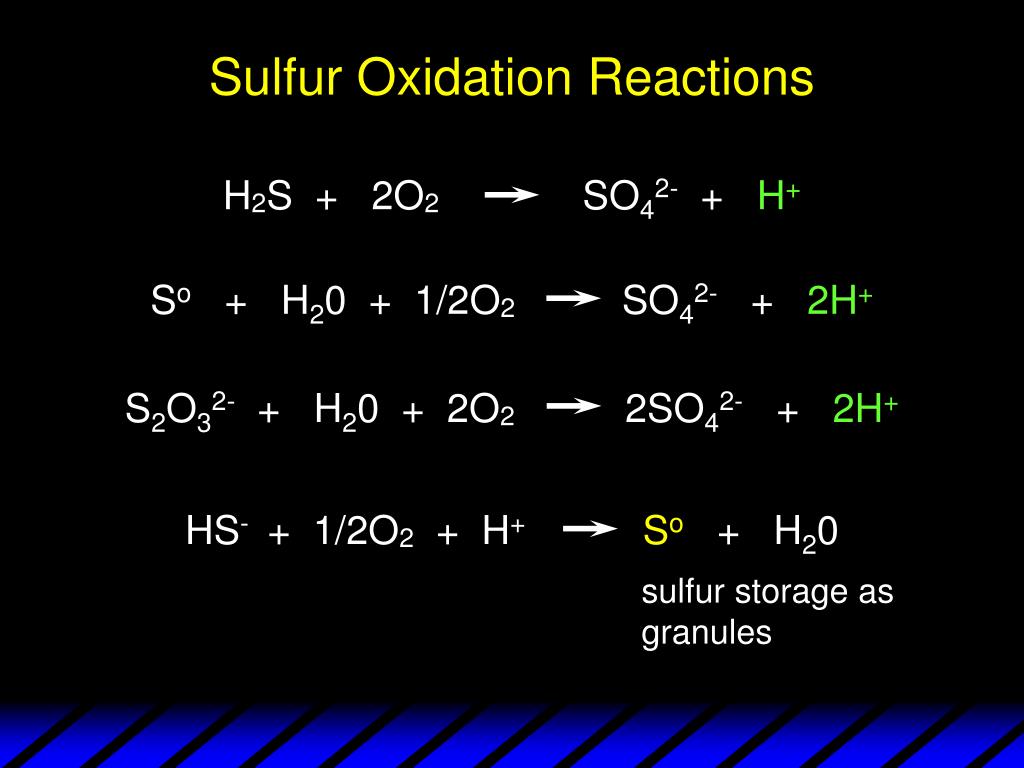
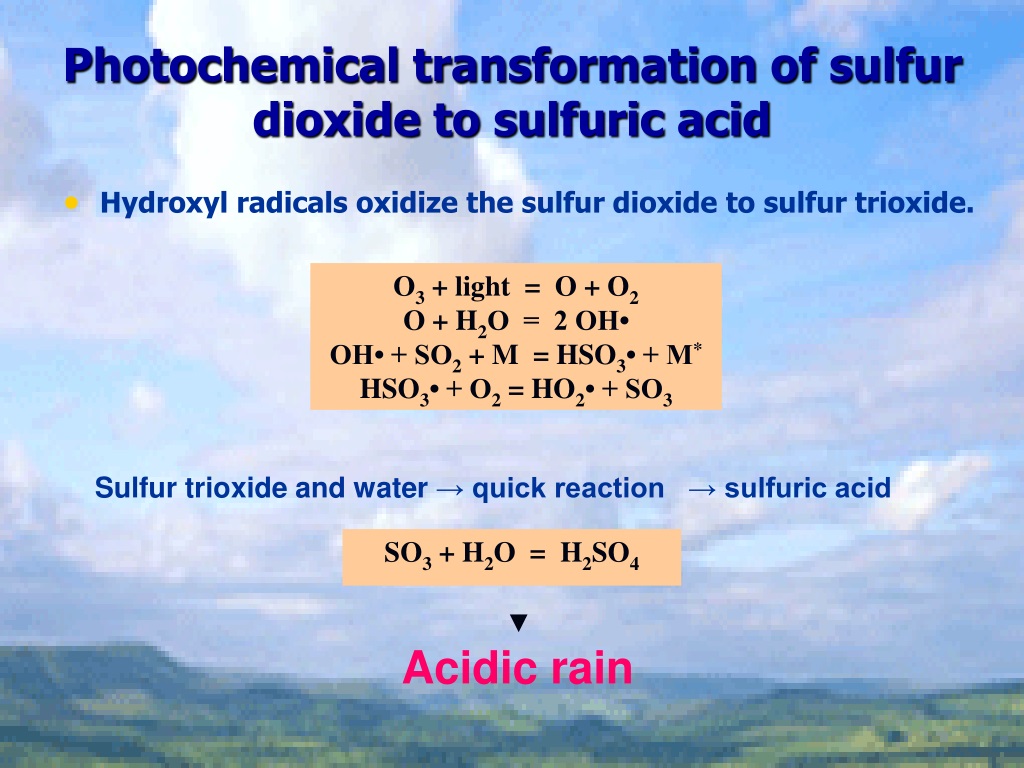
In sulfuric acid, we've got hydrogen (H), sulfur (S), and oxygen (O) all working together. These are atoms with very different electronegativity.
Think of it as a tug-of-war for electrons. Oxygen is the strongest player here, always hogging electrons, which means it usually has an oxidation number of -2. Hydrogen, on the other hand, likes to give away its electron, making it +1.
And now, for the big reveal. Since the entire molecule of H2SO4 is neutral (no overall charge), all the oxidation numbers have to add up to zero. This is like following a budget!
The Math Behind the Magic
We have two hydrogens, each with a +1 charge. This gives us a total of +2 from the hydrogens. It's as if they are donating their electrons!
Then we have four oxygens, each with a -2 charge. That's a whopping -8 coming from the oxygens. Think of them as electron vampires!
So, what number do we need for sulfur to balance everything out and reach that magical zero? Here's the equation: (+1 * 2) + (S) + (-2 * 4) = 0
Simplifying, we get: 2 + S - 8 = 0. This quickly converts to S - 6 = 0. Solving this final equation you will find out the magic number!
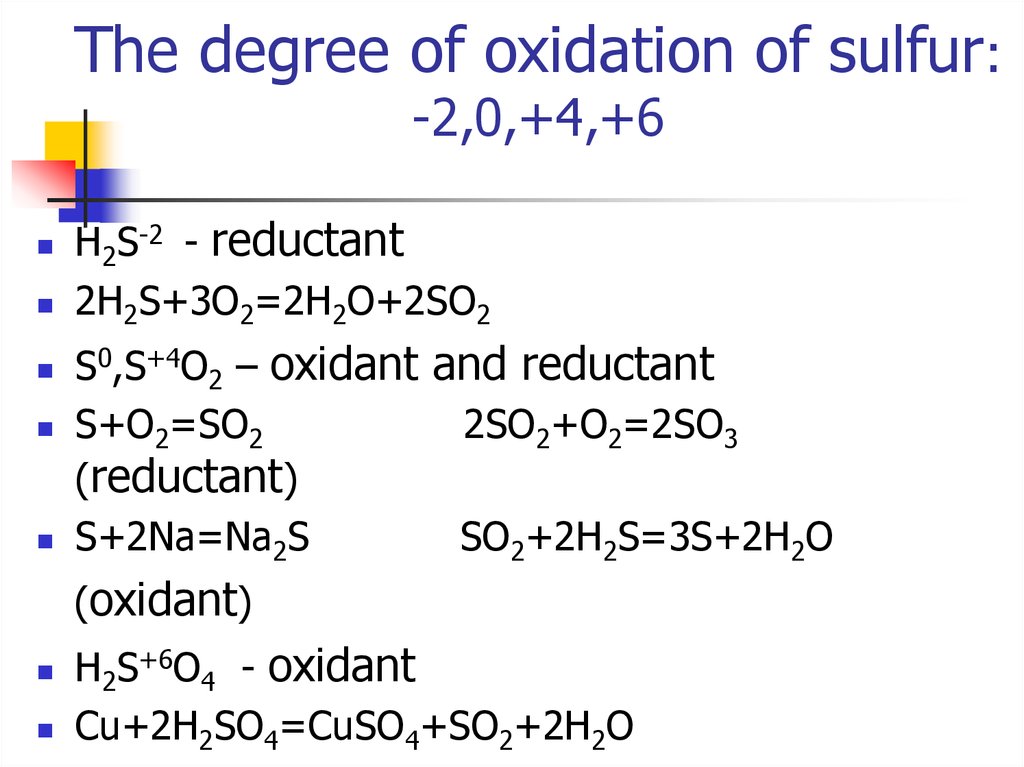
That's right! Sulfur's oxidation number in sulfuric acid is +6. It's lost six electrons (in a pretending sort of way) to its greedy oxygen neighbors.
Why Bother Knowing This?
Okay, so maybe memorizing oxidation numbers isn't your idea of a fun Saturday night. But understanding them is like unlocking a secret code to how chemical reactions work!
It tells you how electrons are being transferred, and that's crucial in understanding everything from batteries to the rusting of metal. Plus, you can impress your friends at parties! (Just kidding... mostly.)
Think of it like this: if you know sulfur's oxidation number, you have a better understanding of what kind of shenanigans it might get up to with other chemicals. Knowing what kind of "shenanigans" to expect might just save the day.
"Chemistry can be a good and bad thing. Chemistry is good when you make love with it. Chemistry is bad when you make crack with it." - Adam Sandler
So next time you see sulfuric acid, remember our friend sulfur with its +6 oxidation number. It's a tiny, unassuming atom with a surprising amount of power... at least, in the world of chemical bonding!