What Is The Oxidation State Of Sulfur In H2so4
Sulfuric acid, H2SO4, it sounds intimidating, right? Like something a mad scientist brews up in a bubbling cauldron. But trust me, understanding one small thing about it, its oxidation state, can be surprisingly... well, not *exciting* exactly, but definitely less scary!
The Case of the Curious Sulfur
Imagine sulfur as a little social butterfly atom. It loves to mingle with other atoms, sharing and borrowing electrons. The oxidation state is basically a way to keep track of how many electron friends sulfur has made, or, more accurately, *how it thinks* it's made.
Think of it like this: sulfur walks into a potluck dinner. It sees hydrogen, known for always bringing a dish that's “slightly” lacking electrons (positive charge!). Then it spots oxygen, a notorious electron hog (negative charge!).
Hydrogen's Humble Contribution
Each hydrogen (H) brings a positive charge ( +1) to the party. And we have two of them, so that's +2 total. They are always generous.
Oxygen's Greedy Grab
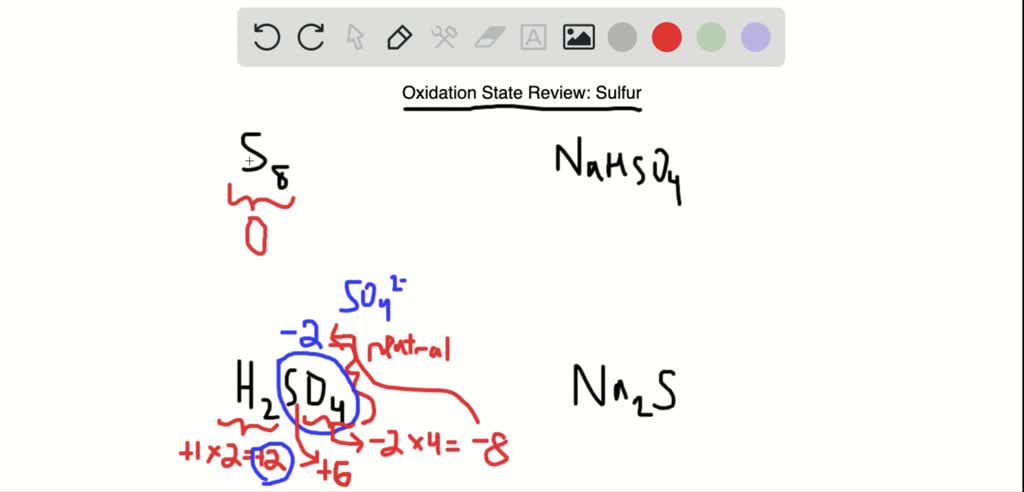
Now, oxygen (O) is a real electron magnet, with a negative charge (-2). Sulfuric acid has four of these, making it -8. Oxygen is a bit of a bully, always taking the lion's share.
Sulfur's Balancing Act
Here's where our friend sulfur comes in. It needs to balance the whole thing out, like a responsible host ensuring there’s enough cake for everyone! The entire molecule has to be neutral, meaning the charges all have to cancel each other out.
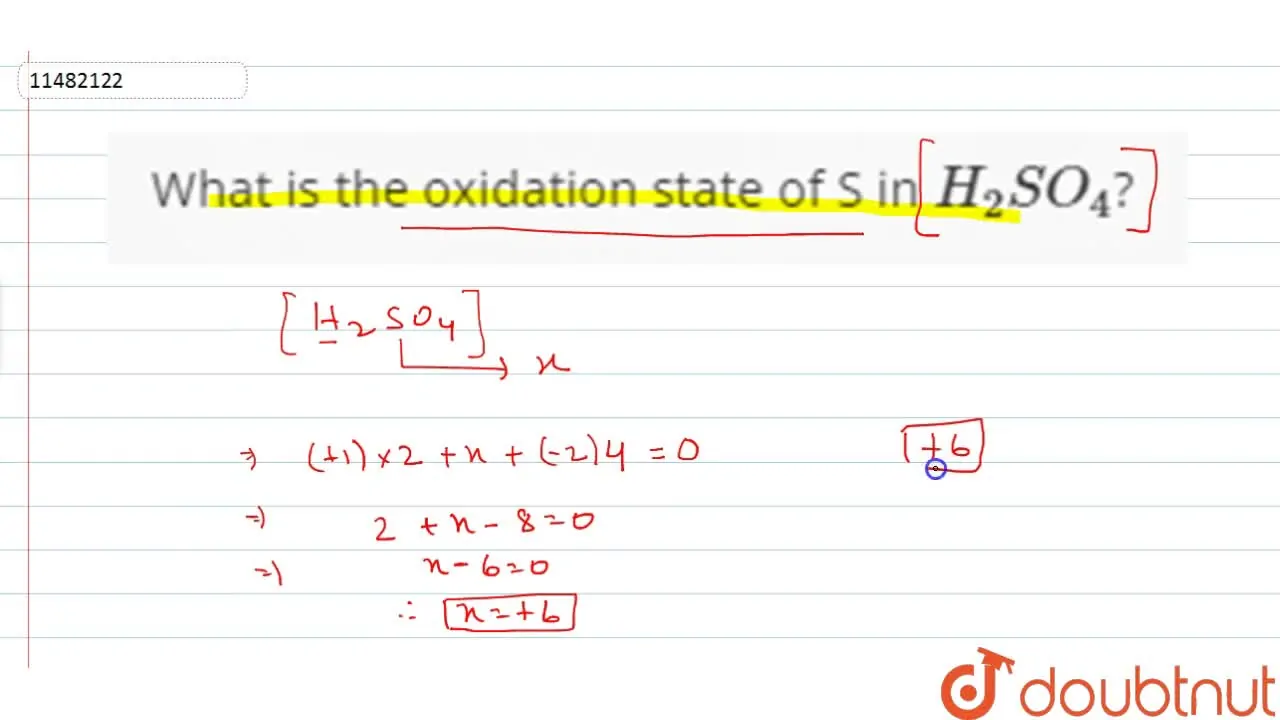
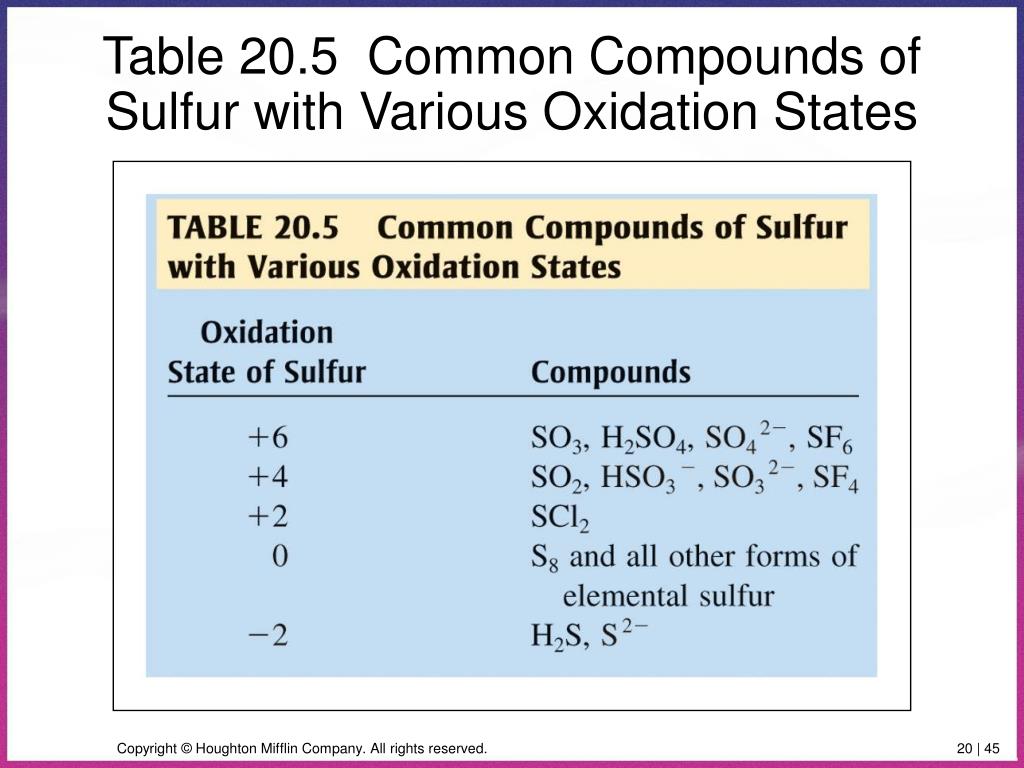
So, we have +2 from hydrogen and -8 from oxygen. What number would sulfur need to be to make it all add up to zero? Let's call sulfur's oxidation state "x". The equation is: +2 + x - 8 = 0. Solving for x, we get x = +6. That's sulfur oxidation state!
Sulfur: The +6 Dynamo
So, the oxidation state of sulfur in H2SO4 is +6. This means sulfur is essentially donating six electrons in its bonds. It might sound complicated, but really, it's just about balancing the books in the atomic world.
It is like sulfur lends out all these electrons and feels a little bit emptier, like a generous friend after giving the last slice of pizza to someone else. But without it, there is no pizza party!
Now, armed with this knowledge, you can casually drop into conversation at your next dinner party, "Did you know the oxidation state of sulfur in sulfuric acid is +6?" You'll be the hit of the evening, guaranteed! (Okay, maybe not guaranteed, but you'll definitely have something interesting to say.)
Why Bother?
You might be thinking, "Okay, that's mildly interesting, but why should I care?" Well, understanding oxidation states helps us predict how chemicals will react with each other. It's like knowing which ingredients will cause a baking disaster and which will create a delicious cake.
Sulfuric acid, thanks to sulfur's +6 oxidation state, is a powerful player in many chemical reactions. From manufacturing fertilizers to cleaning drains (safely, of course!), it's all about how those electron interactions work.
Next time you see sulfuric acid mentioned, don't just think of bubbling beakers and dangerous chemicals. Remember the social butterfly sulfur, diligently balancing the electron books and making the chemical world a more interesting place. You might even smile a little.

+Determine+the+oxidation+state+of+sulfur+in+each+of+the+following:+a)+H2S..jpg)