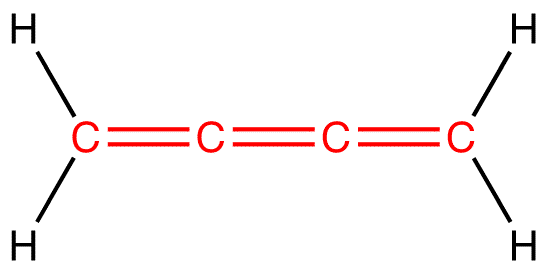
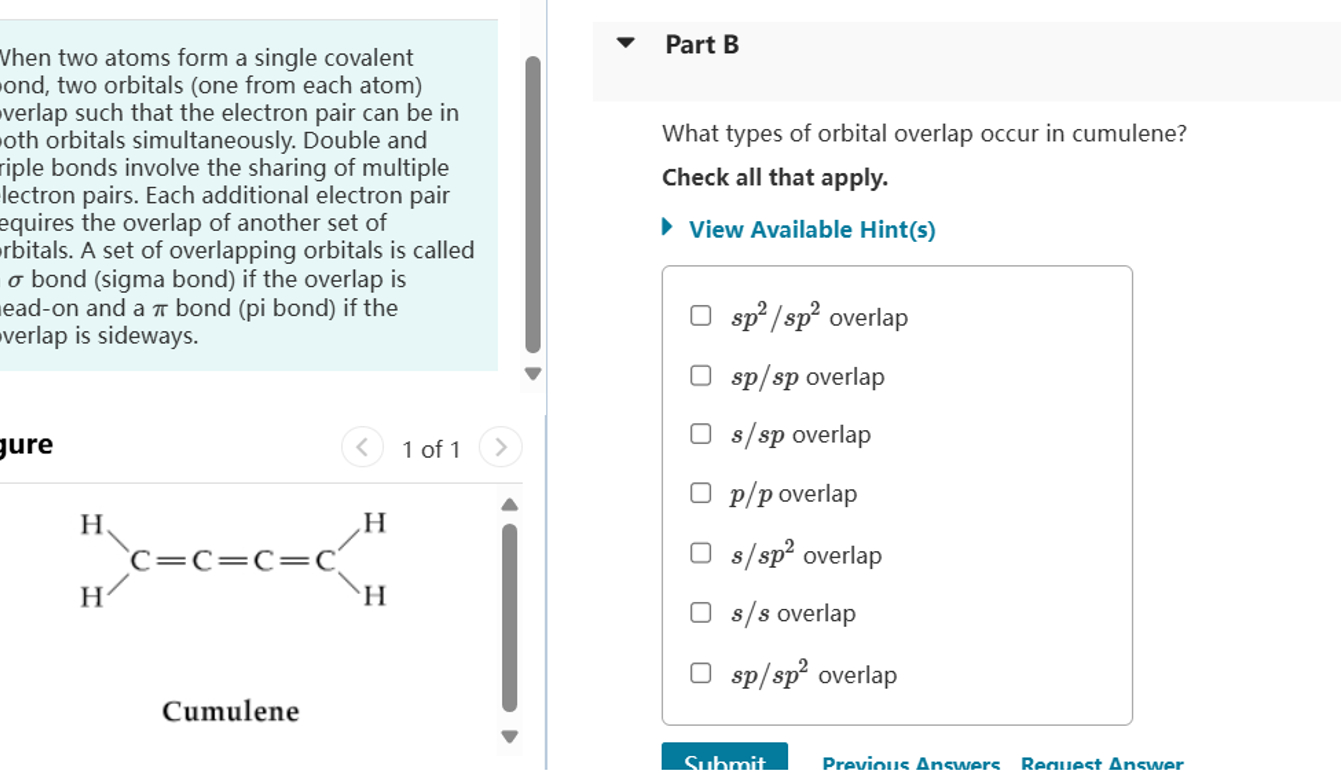
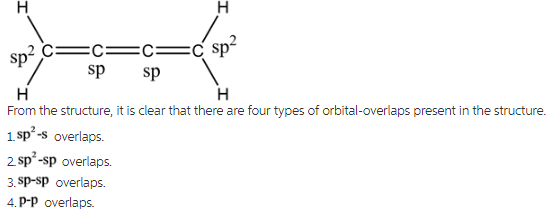
What Types Of Orbital Overlap In Cumulene
Have you ever played with a Slinky, that classic toy that walks down stairs with a mesmerizing wobble? Well, imagine stretching that Slinky out, making it incredibly long, and building it out of carbon atoms. That, in a very basic way, gives you a glimpse into the fascinating world of cumulenes!
Cumulenes: The Chemical Centipede
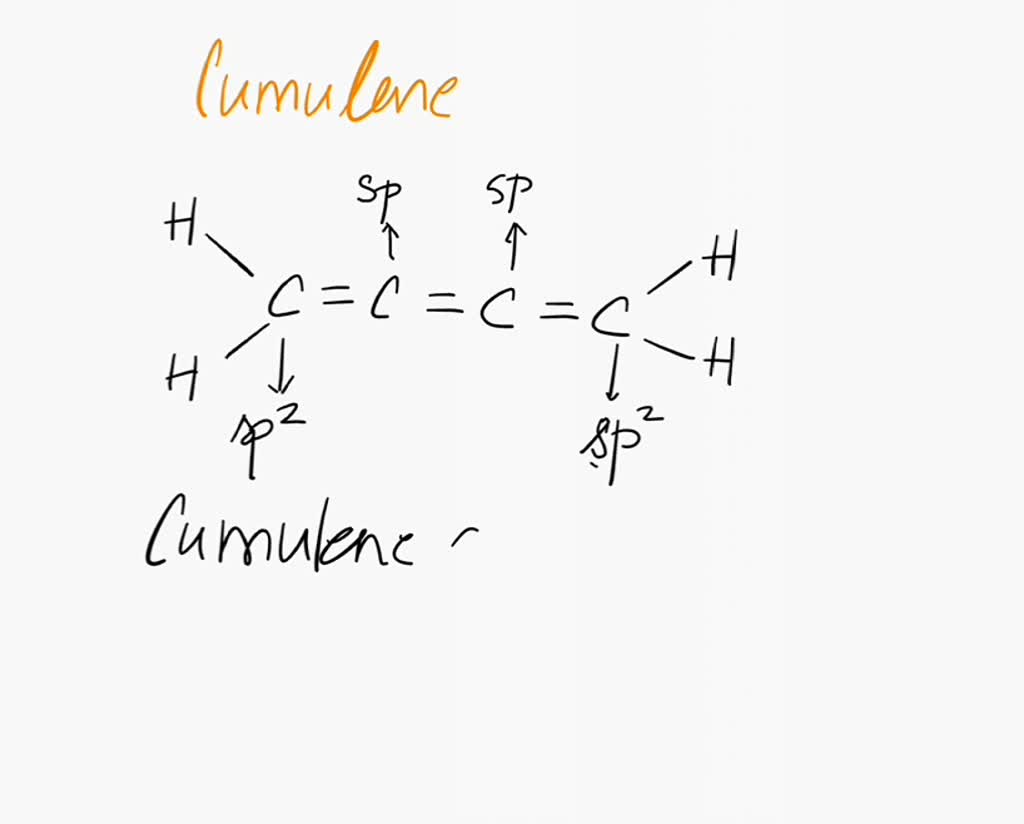
Cumulenes are molecules that are basically long chains of carbon atoms linked together by double bonds. Think of each double bond as two friends holding hands – a strong connection. The real magic of cumulenes, and what makes them different from other carbon chains, lies in how those "hand-holds" arrange themselves.
These hand-holds, scientifically called *pi bonds*, are actually formed by the *overlap of atomic orbitals*. It's like two shy dancers trying to hold hands without actually looking at each other directly. Awkward, right? But also kind of elegant!
Sigma and Pi: The Dynamic Duo
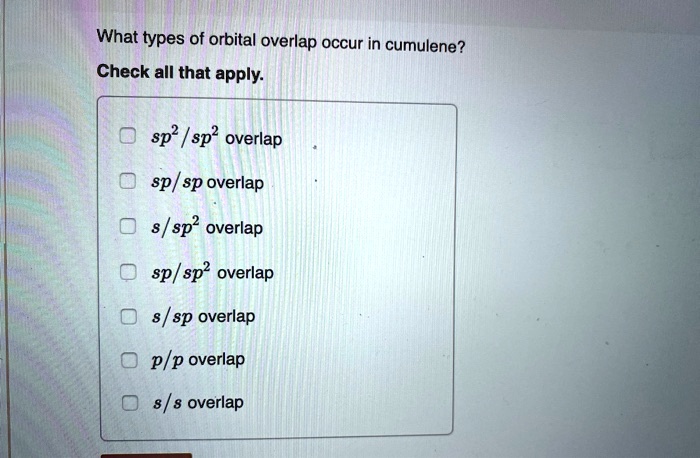
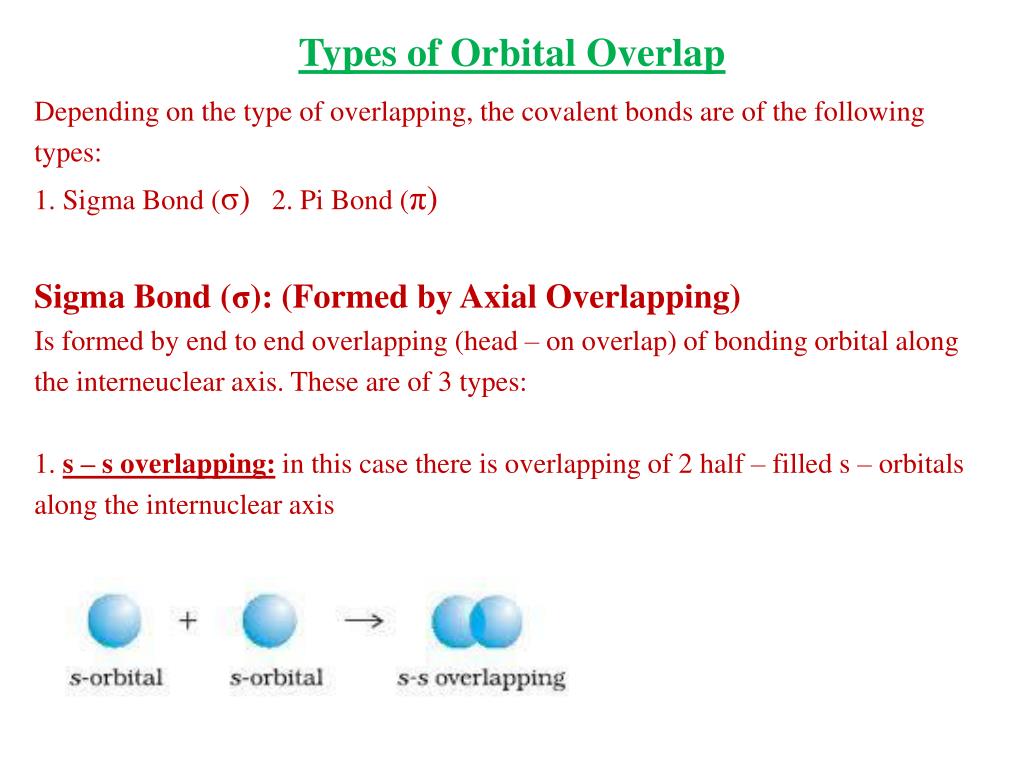
Let's break down the types of orbital overlap. First, we have the *sigma bond*, which is the strong, direct handshake. It's formed by orbitals overlapping head-on. Think of it as a confident, firm grip.
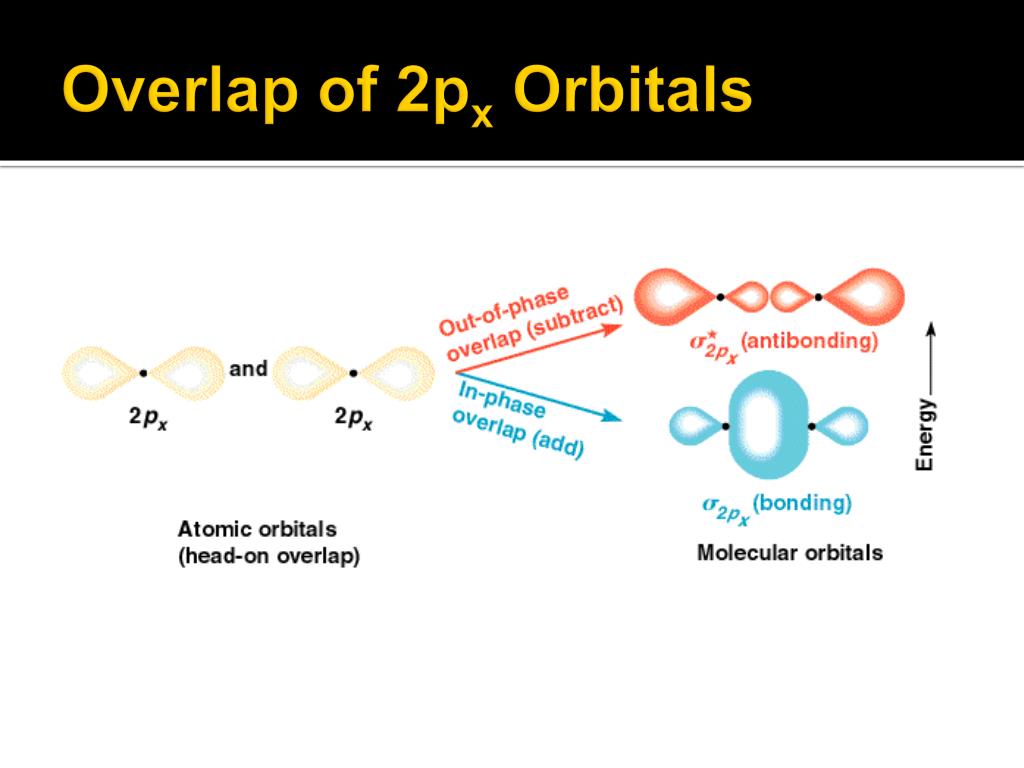
Then we have the more subtle *pi bond*, formed by the sideways overlap of orbitals. It's a weaker, more tentative connection, but just as important. Like that shy dancer, this bond is crucial for the cumulene's unique personality.
The Overlap Tango: p-orbitals
These pi bonds use special orbitals called *p-orbitals*. Visualize them as dumbbells sitting on each carbon atom. Each p-orbital is ready to reach out and overlap with the p-orbital of its neighbor, creating that sideways "hand-hold."
Now, here's where the fun begins. With consecutive double bonds, these p-orbitals have choices! They can align in the same plane, or they can twist and align in a perpendicular plane for each consecutive carbon atom.
Even vs. Odd: A Cumulene Personality Test
The number of carbon atoms in the chain dictates the cumulene's personality. If you have an *even* number of carbons, the p-orbitals tend to align in two different planes. Imagine holding your hands out in front of you, one above the other, like you are holding a beach ball. That's the rough idea!
This gives the cumulene a linear shape, like a stretched-out Slinky. It's straight, rigid, and ready to party, showing interesting optical properties because of this arrangement.
But if you have an *odd* number of carbons, all the p-orbitals try to squeeze into the same plane. That will twist the ends. Imagine trying to force a very long ribbon to lie perfectly flat on a table. The ends start to bend, right? That's what happens to odd-numbered cumulenes!
Twisted Tales and Molecular Chirality
This twisting can lead to *chirality*, where the molecule and its mirror image are non-superimposable. Like your left and right hands! Isn't it wild to think that a simple chain of carbon atoms can exhibit this kind of complexity?
So, next time you see a picture of a cumulene, don't just see a bunch of lines and letters. Think of the shy p-orbitals, the dynamic dance of sigma and pi bonds, and the remarkable influence of even and odd numbers. You might just find yourself looking at a tiny, molecular Slinky with a whole new appreciation!
Perhaps cumulenes aren't so different from us. Maybe the way they bond, twist, and interact is a tiny reflection of the way we connect with each other, reaching out with both strong handshakes and tentative sideways glances.
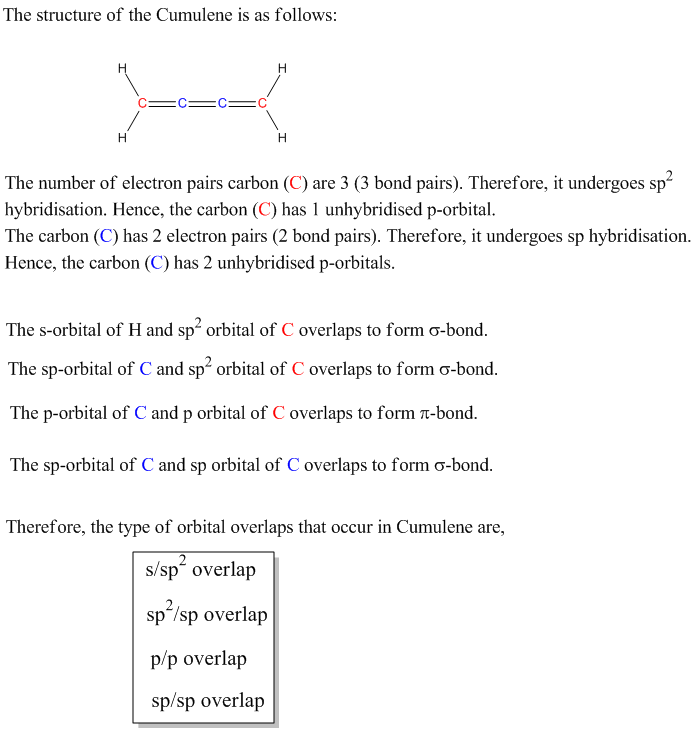








.jpg)