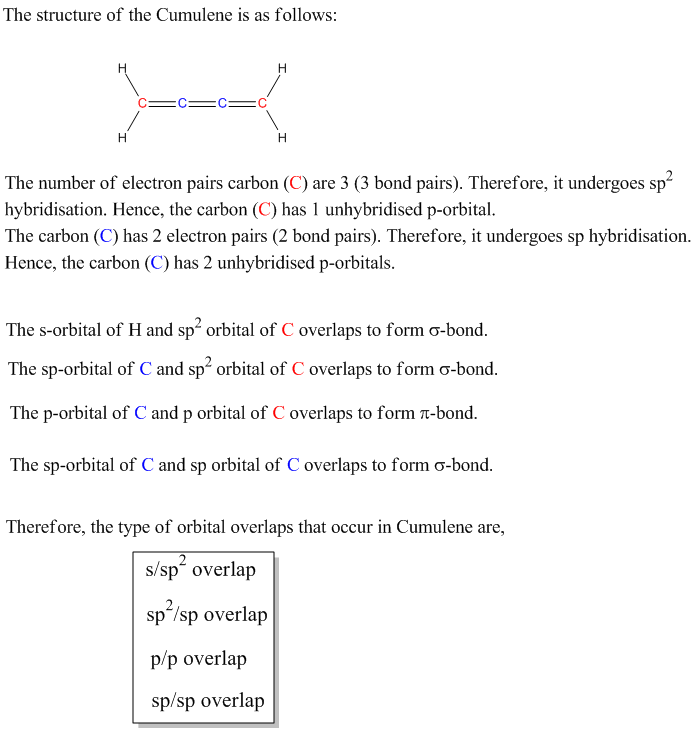
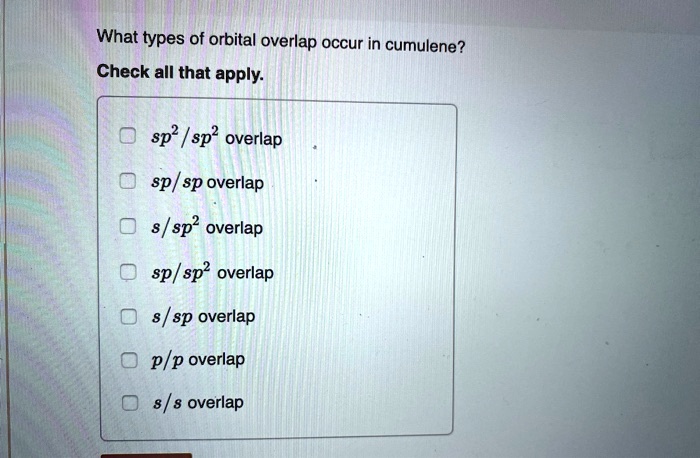
What Types Of Orbital Overlap Occur In Cumulene
Cumulenes! Sounds like something out of a sci-fi movie, right? Well, these molecules are very real, and they have a fascinating secret: their orbital overlap. Let's dive into what makes them so special.
What's So Cool About Cumulenes?
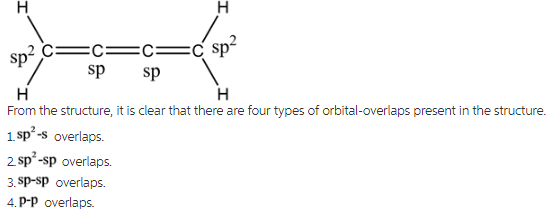
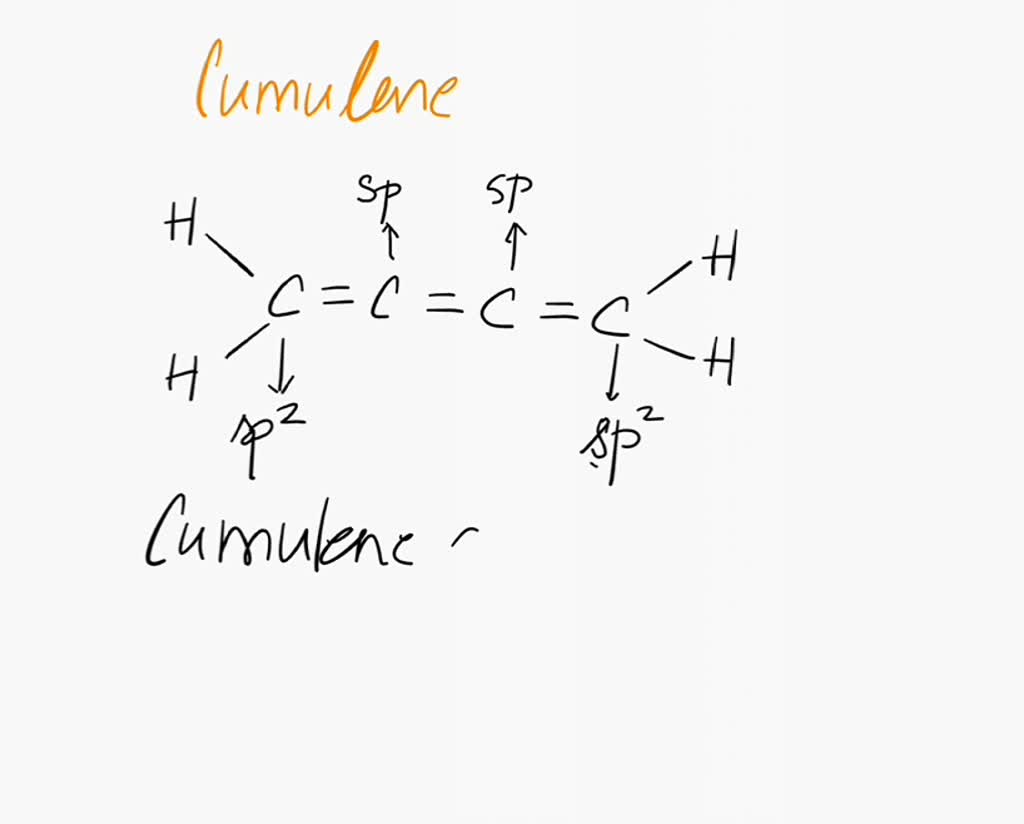
Imagine a carbon chain holding hands, not just one hand, but *two*! That's essentially what a cumulene is. They're hydrocarbons with a string of consecutive double bonds.
Each carbon atom in the chain is linked to its neighbor by two bonds, instead of one single bond. This arrangement gives cumulenes their unique characteristics.
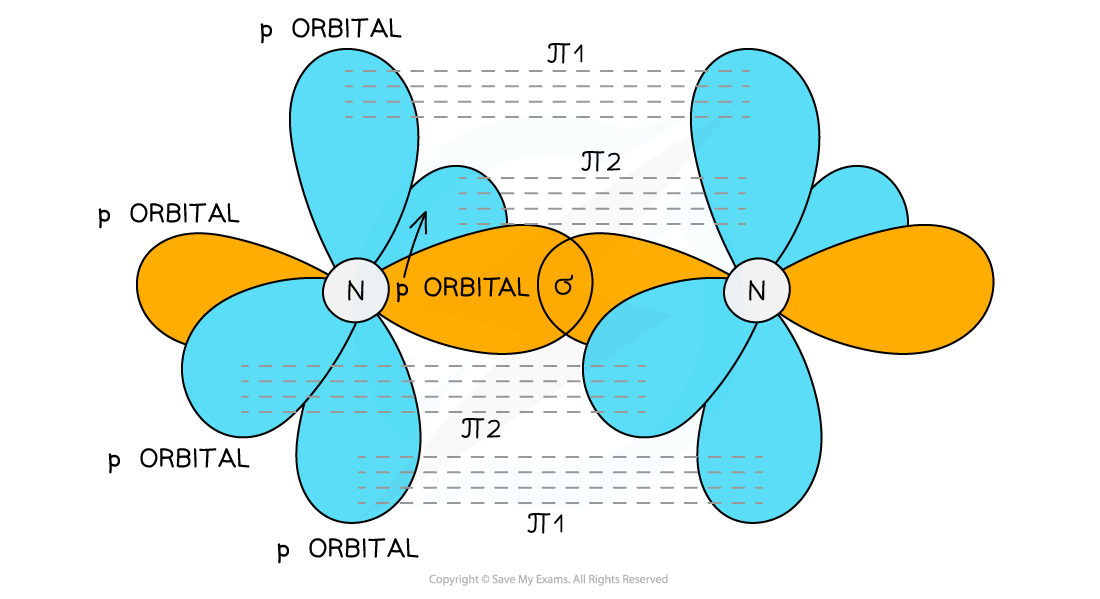
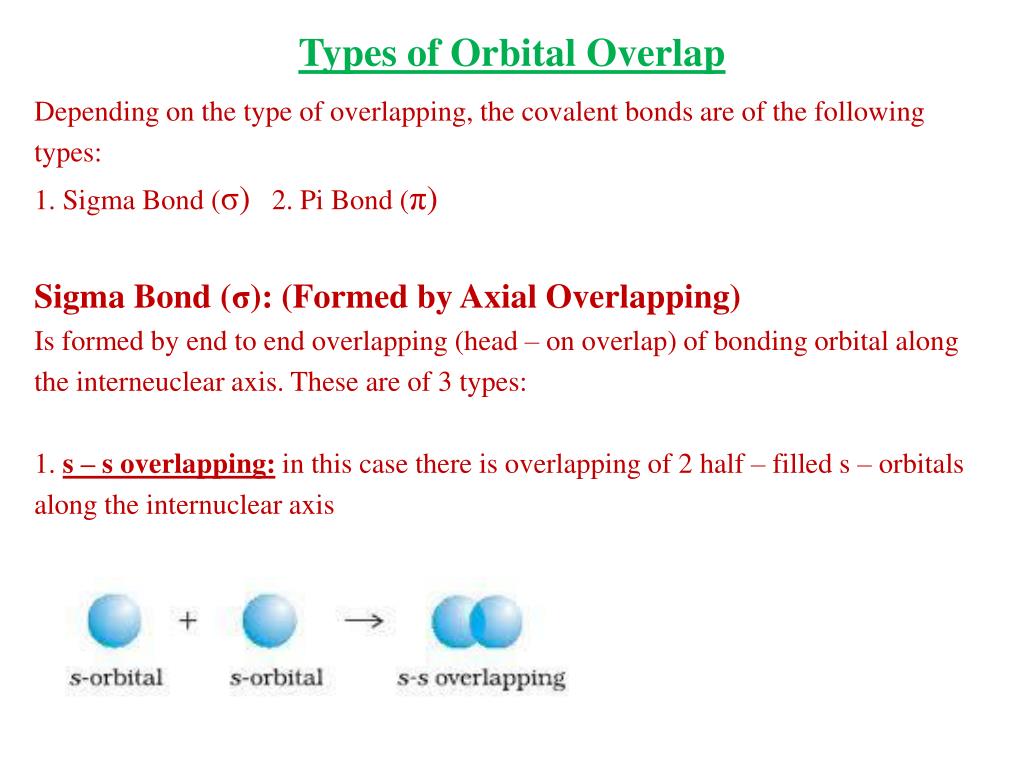
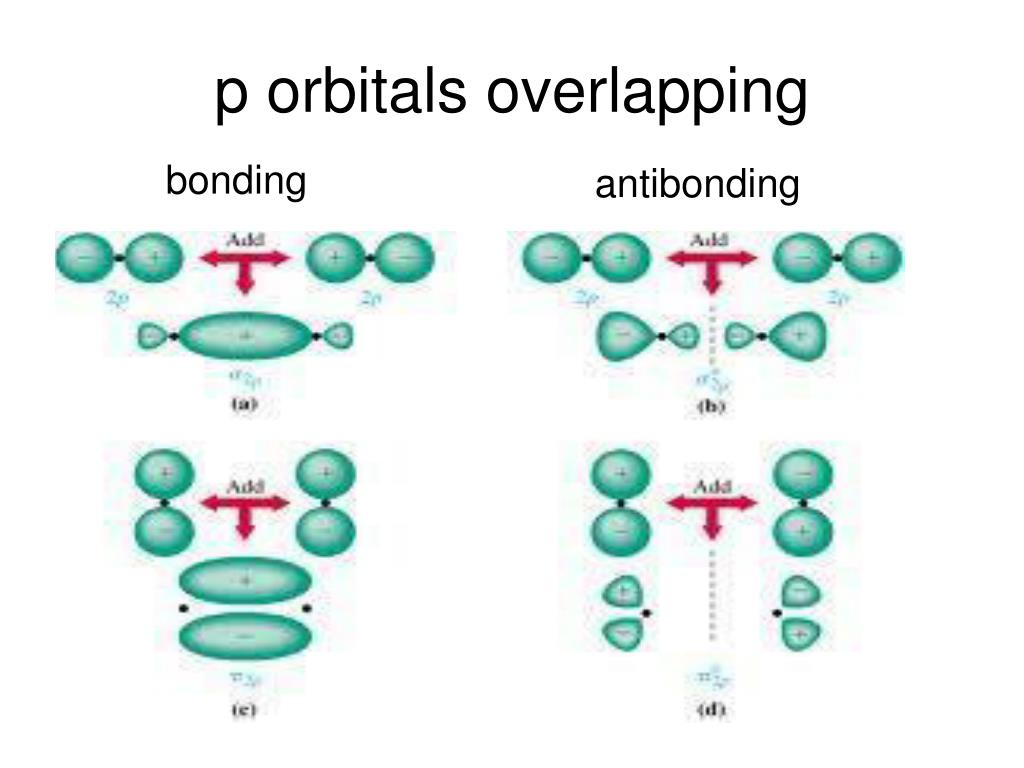
The Key Players: Sigma (σ) and Pi (π) Bonds
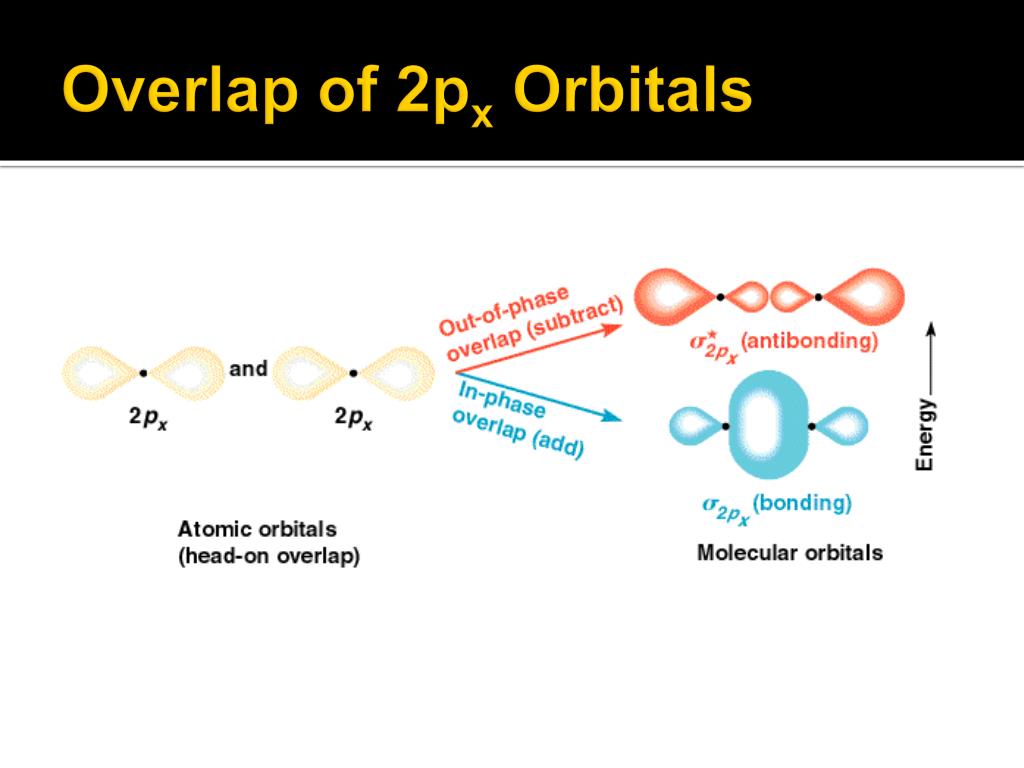
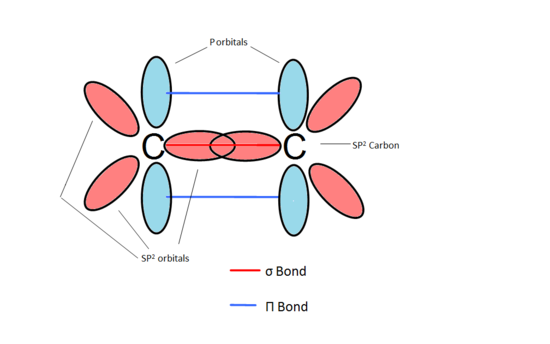
To understand orbital overlap in cumulenes, we need to meet two important characters: sigma (σ) bonds and pi (π) bonds. Think of sigma bonds as the strong, central connection. It's like the spine of the bond.
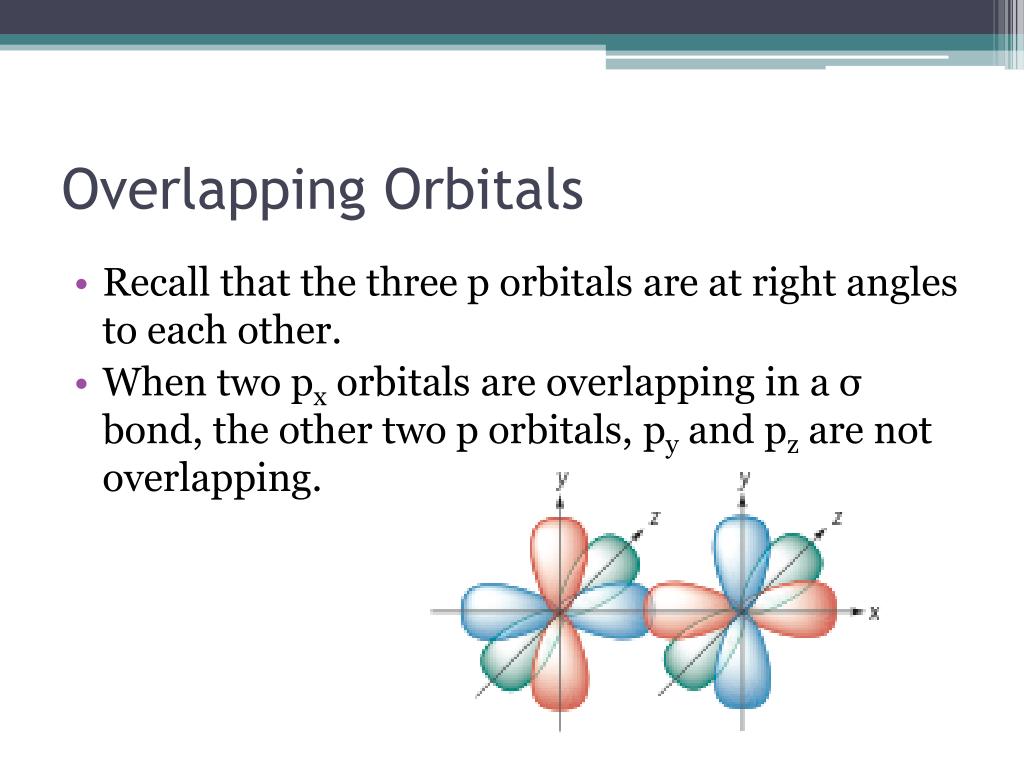
Pi bonds are the sidekicks, adding extra bonding strength. They are formed by overlapping p-orbitals above and below (or to the sides) of the sigma bond. These bonds dictate much of the molecule's behavior.
Orbital Overlap: The Dance of Electrons
Orbital overlap is where the magic happens. It describes how atomic orbitals combine to form molecular orbitals, essentially creating the bonds that hold the molecule together. Electrons like to hang out where orbitals overlap. This stabilizes the molecule.
In cumulenes, the story gets interesting. Because of the consecutive double bonds, the pi bonds don't all line up the same way. This leads to some pretty wild consequences.
The Twisting Tale of Cumulenes
Even-numbered cumulenes (those with an even number of double bonds) are fascinating. Their end groups end up on different planes. Think of it like twisting a ribbon.
This twisting is all thanks to the pi bonds doing their own thing. The first pi bond is perpendicular to the second. This forces the groups at the ends of the chain to be perpendicular to each other too!
Odd-numbered cumulenes are different. The groups are on the same plane. Think of it like a normal flat molecule.
Why Should You Care?
Okay, so molecules are twisting, big deal, right? Wrong! This unique structure has a massive impact on how cumulenes behave. It affects their chemical reactivity and even their optical properties!
The unique shapes created by orbital overlap can lead to very special applications. Scientists are studying them for use in advanced materials, molecular electronics, and other cool technologies.
More Than Just a Pretty Molecule
Cumulenes aren't just theoretical curiosities. They've been found in space! So next time you look up at the stars, remember these twisted molecules are out there too.
Understanding their orbital overlap helps us understand the very building blocks of the universe. That's pretty amazing, wouldn't you agree?
So, next time you’re looking for a fascinating topic to explore, consider the wonderful world of cumulenes. It's a twisty, turny adventure into the heart of chemistry! Maybe you will read more about Ethene, Allene and Butatriene.
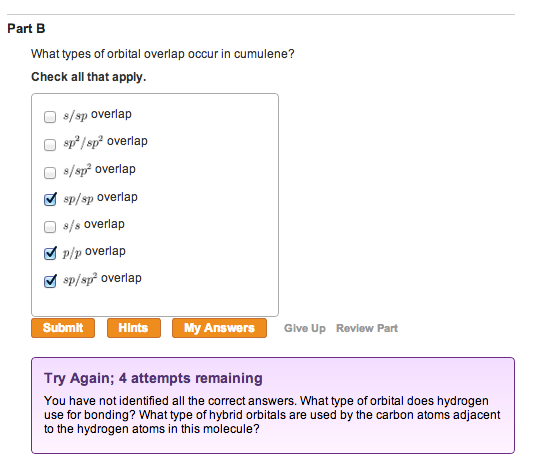





![What Types Of Orbital Overlap Occur In Cumulene Optimized structure and bonding π molecular orbitals of [4]cumulene](https://www.researchgate.net/publication/324762597/figure/fig5/AS:619482464649225@1524707537940/Optimized-structure-and-frontier-p-molecular-orbital-pairs-of-4cumulene-pentatetraene_Q640.jpg)