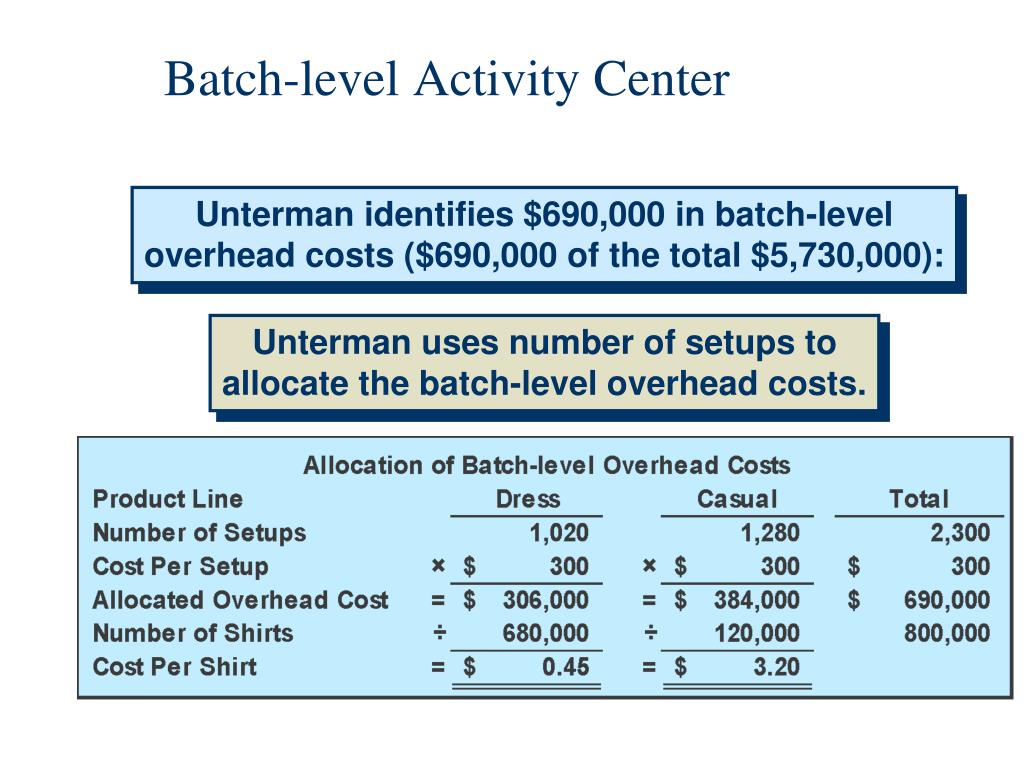
A Batch Level Activity Will Vary With The

Urgent warnings are being issued across multiple industries as a critical flaw impacts batch level activities. Production lines and quality control processes are facing immediate disruptions.
This issue, identified as the unpredictable variance in batch-level activities, throws into question the reliability of standardized processes. The ripple effect is already being felt, triggering emergency meetings and frantic re-evaluations across sectors including pharmaceuticals, food production, and manufacturing.
Widespread Impact Reported
The problem stems from an unforeseen instability within previously reliable batch processing systems. The exact cause remains under investigation, but the effects are undeniably widespread and potentially catastrophic.
Pharmaceutical companies are scrambling to reassess drug batches. Concerns revolve around the consistency of active ingredients, dosage accuracy, and potential adverse reactions due to the variability.
Food production facilities are facing similar anxieties. Ensuring consistent taste, texture, and safety of processed foods is now a major challenge.
Manufacturing plants are experiencing production delays. Faulty components and inconsistent product quality are leading to significant setbacks and financial losses.
Specific Examples Surface
Acme Pharmaceuticals issued a recall of its flagship allergy medication on Tuesday. Preliminary tests revealed inconsistent potency levels across different batches.
The Food and Drug Administration (FDA) is actively investigating the situation at Acme's manufacturing plant in Newark, New Jersey.
Global Foods Inc. halted production of its popular frozen dinners late yesterday. Customer complaints about varying tastes prompted an internal investigation, revealing inconsistencies in the seasoning blend process.
A spokesperson for Global Foods stated, "We are committed to ensuring the highest quality and safety standards. Production will resume once we identify and rectify the source of the inconsistency."
Precision Motors, a key supplier for the automotive industry, reported significant defects in a batch of engine components. The company's stock price plummeted after the announcement.
Investigation Underway
The source of the batch-level activity variance is currently unknown. Experts are pointing to several possible factors, including subtle changes in raw material composition, equipment malfunction, and unforeseen interactions within the processing environment.
A team of engineers and scientists from the National Institute of Standards and Technology (NIST) is assisting in the investigation. They are focusing on identifying the root cause and developing mitigation strategies.
Early reports suggest that even minor deviations in environmental conditions, such as temperature or humidity, can significantly impact batch consistency. This necessitates a thorough review of existing control measures.
Immediate Actions Required
Companies across all affected sectors are urged to immediately reassess their batch processing procedures. Thorough testing and quality control measures are paramount to identify and isolate any potential issues.
The FDA has issued specific guidelines for pharmaceutical companies, emphasizing the need for rigorous batch-to-batch testing and detailed documentation. Non-compliance could result in severe penalties.
Manufacturing facilities should implement enhanced monitoring systems to track environmental parameters and material properties. Early detection is crucial to prevent further disruptions.
Looking Ahead
The investigation is expected to continue for several weeks. Regular updates will be provided by NIST and other regulatory agencies.
The long-term impact of this incident remains uncertain. However, it has already exposed vulnerabilities in existing quality control systems. Robust monitoring, rigorous testing, and adaptable processing strategies will be essential to prevent similar incidents in the future.
Businesses should prioritize transparency and communication with their stakeholders. Openly addressing concerns and implementing corrective actions will be vital to rebuilding trust and ensuring consumer safety.







:max_bytes(150000):strip_icc()/batch-level-activities.asp_Final-ddfa8cff26ea40efb48fda57f9d7b87c.png)

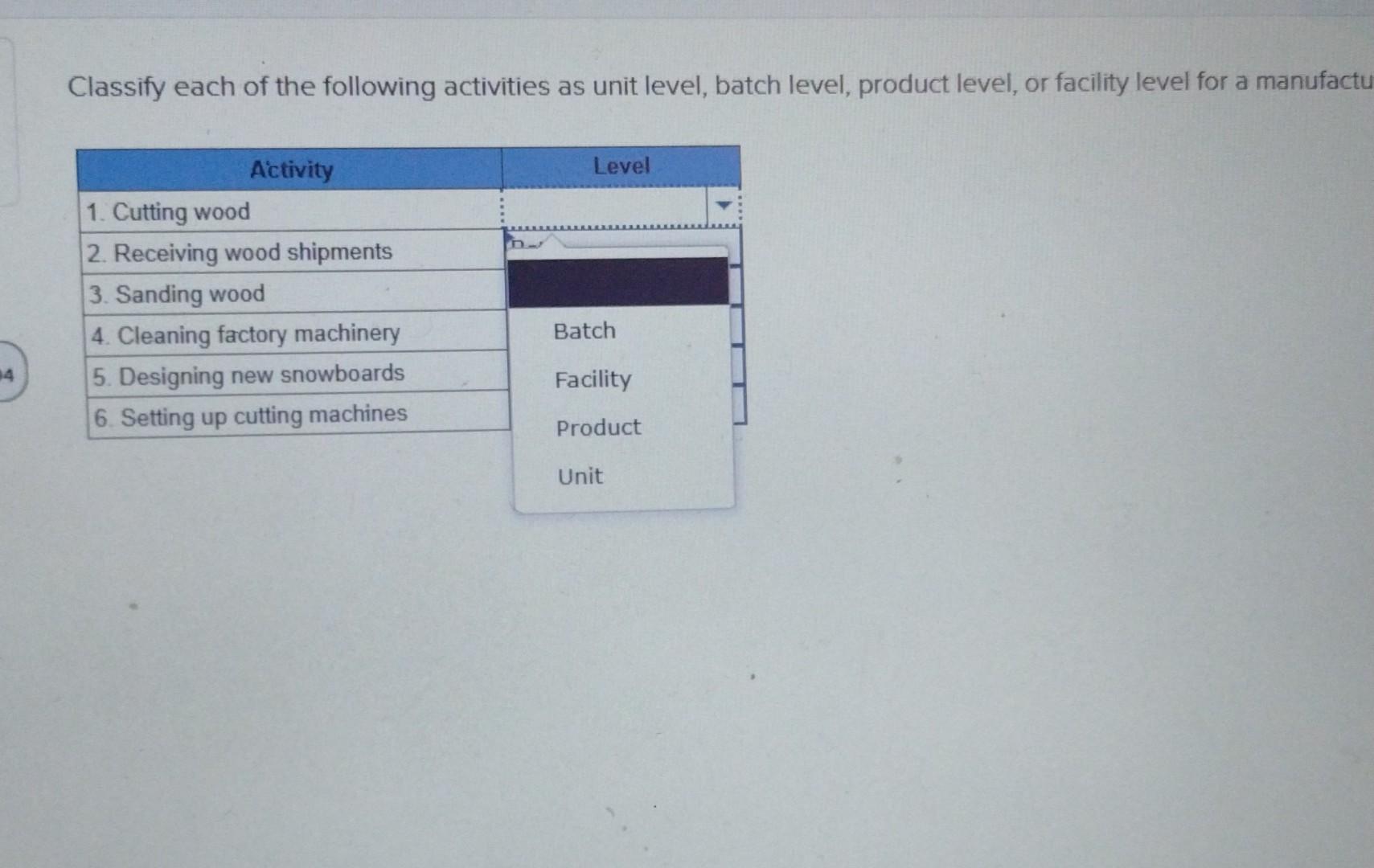

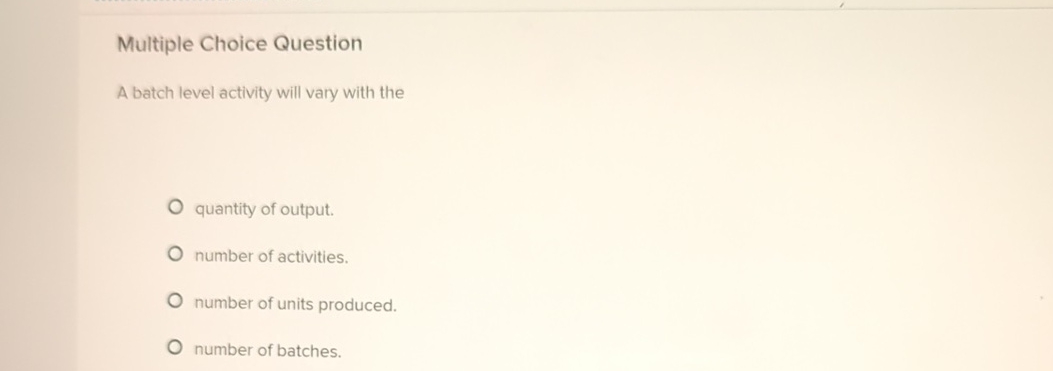
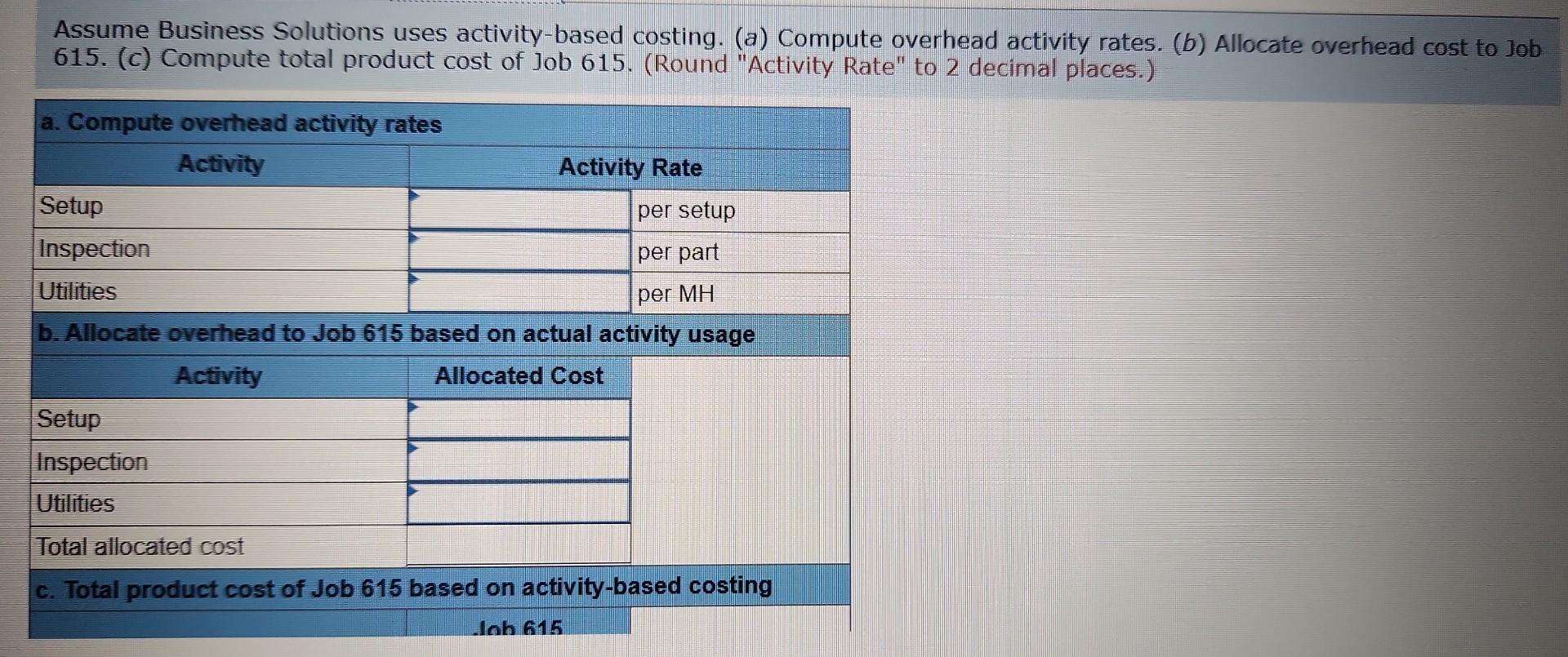

+Learning+Objective+C3:+Describe+the+four+types+of+activities+that+cause+overhead+costs..jpg)