Abandoned In The Middle Of Clinical Trials

For hundreds of individuals battling debilitating diseases, clinical trials represent a beacon of hope, a chance at a better life, or perhaps even survival. But what happens when that light is abruptly extinguished, leaving participants stranded mid-treatment?
This is the increasingly concerning reality for many involved in clinical trials that are prematurely terminated due to funding shortfalls, strategic shifts by pharmaceutical companies, or unforeseen regulatory hurdles. The sudden cessation of these trials raises serious ethical questions about the obligations owed to participants who have placed their trust, and often their well-being, in the hands of researchers and sponsors.
The Growing Problem of Abandoned Trials
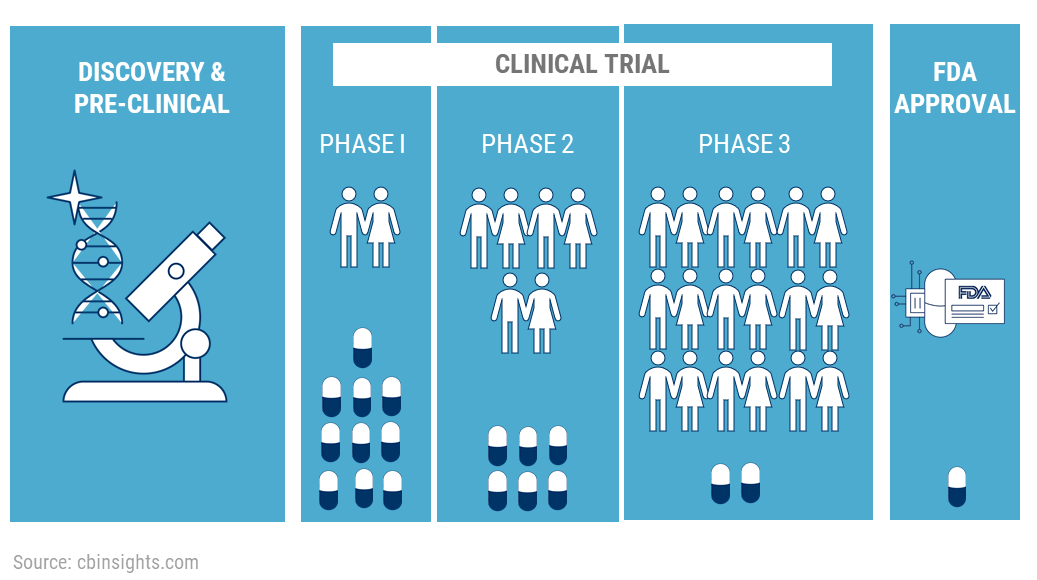
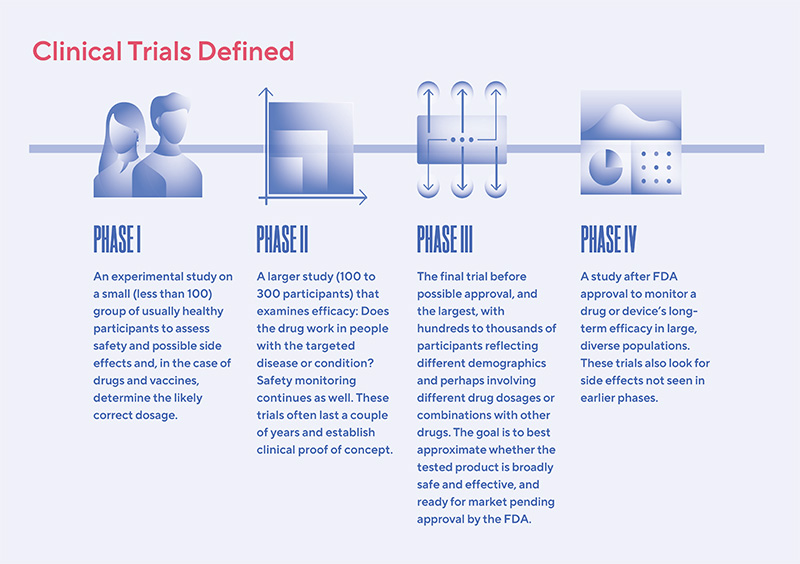
Clinical trial abandonment, while not a new phenomenon, is gaining greater attention as the number of trials increases and the financial pressures on the pharmaceutical industry intensify. A 2018 study published in JAMA Internal Medicine estimated that roughly 8% of registered clinical trials are terminated early. While not all terminations constitute abandonment – some are halted due to safety concerns or clear efficacy – a significant portion leave participants without a clear plan for continued care.
The impact on participants can be devastating. Many enroll in trials as a last resort, after exhausting conventional treatment options. They may experience significant physical and emotional distress when the treatment they've come to rely on is suddenly withdrawn. Furthermore, the loss of access to specialized medical care and monitoring associated with the trial can have long-term health consequences.
Who Is Affected?
The individuals affected span a wide range of demographics and diseases. Oncology trials, which often involve complex and expensive treatments, are particularly vulnerable to early termination. Rare disease patients, who have limited treatment options to begin with, are also disproportionately impacted when trials targeting their conditions are abandoned.
Dr. Emily Carter, a bioethicist at the University of Pennsylvania, explains: “Participants in clinical trials are often in a vulnerable position. They’ve agreed to participate in research with the understanding that they will receive some level of care and potential benefit. When that promise is broken, it can have a profound impact on their lives.”
Case Studies in Abandonment
In 2021, BioSolve Therapeutics, a small biotech company, abruptly halted its Phase II trial for a novel cancer drug after failing to secure additional funding. Participants, many of whom had shown initial positive responses to the treatment, were left scrambling to find alternative options. The company offered limited assistance in transitioning patients to other therapies.
Another example involved a Phase III trial for a gene therapy targeting a rare genetic disorder. Genexis Pharma, the sponsoring company, was acquired by a larger pharmaceutical firm that decided to discontinue the trial as it did not align with its long-term strategic goals. Despite promising initial results, participants were cut off from the treatment, and the future of the therapy remains uncertain.
The Ethical and Regulatory Landscape
Current regulations governing clinical trials offer limited protection to participants in the event of trial abandonment. While informed consent documents typically outline the possibility of early termination, they often lack specific details about what will happen to participants in such a scenario.
The U.S. Food and Drug Administration (FDA) provides guidelines for clinical trial conduct but does not mandate specific provisions for continued access to treatment after a trial ends. The National Institutes of Health (NIH) encourages researchers to develop contingency plans for trial termination, but these are not legally binding.
Moving Forward: Potential Solutions
Addressing the issue of clinical trial abandonment requires a multi-faceted approach. Stronger regulatory oversight is needed to ensure that sponsors have adequate financial resources and contingency plans in place before initiating a trial. Researchers should be ethically obligated to develop clear protocols for transitioning participants to alternative care if a trial is terminated.
Establishing a centralized fund to support continued treatment for participants in abandoned trials could provide a crucial safety net. This fund could be supported by contributions from pharmaceutical companies, government agencies, and philanthropic organizations. Increased transparency regarding trial funding and termination decisions would also help protect participants' interests.
Ultimately, protecting the well-being of clinical trial participants is a shared responsibility. Pharmaceutical companies, researchers, regulators, and patient advocacy groups must work together to ensure that individuals who volunteer for these crucial studies are not left behind.