Acetate Of Lime Polar Or Nonpolar

Urgent concerns are mounting regarding the classification of Acetate of Lime (Calcium Acetate). A definitive answer on its polarity—whether it's polar or nonpolar—is critically needed for numerous industrial applications.
The ambiguous nature of Acetate of Lime's polarity is stalling progress in pharmaceuticals, textiles, and food processing, impacting efficiency and potentially safety. Clarification is paramount to avoid costly errors and ensure product integrity.
What is Acetate of Lime?
Acetate of Lime, also known as Calcium Acetate, is a calcium salt of acetic acid. Its chemical formula is Ca(CH3COO)2. It is widely used as a food additive (E263), a stabilizer, and a source of calcium.
It also finds application in dialysis treatments for patients with kidney failure to control phosphate levels. The substance is typically available as a white, odorless powder or crystalline solid.
The Polarity Question: Why Does It Matter?
Polarity dictates how a substance interacts with other compounds. Polar substances dissolve readily in polar solvents like water, while nonpolar substances dissolve in nonpolar solvents like oil.
Understanding the polarity of Acetate of Lime is critical for predicting its solubility, reactivity, and behavior in various chemical processes. Incorrect assumptions can lead to failed experiments, inefficient processes, and potentially hazardous outcomes.
Current Data and Expert Opinions
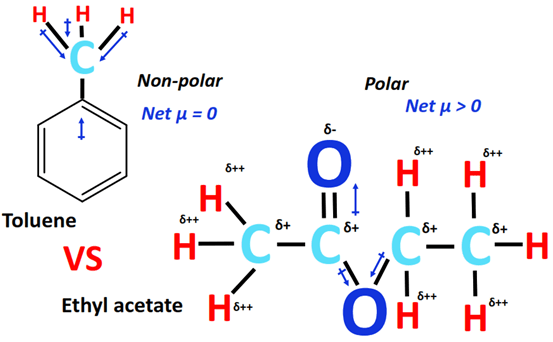
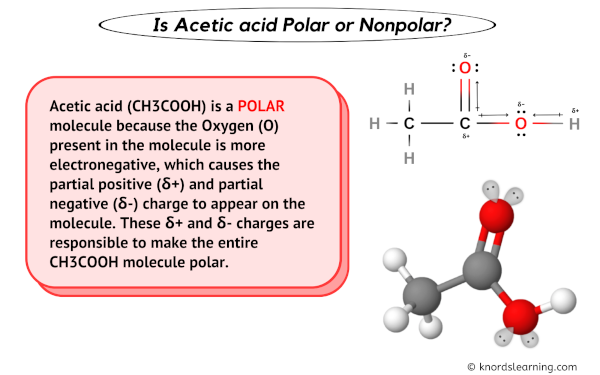
Conflicting data are fueling the controversy. Some sources suggest Acetate of Lime behaves as a polar compound due to the ionic bond between calcium and the acetate ions.
However, other sources point to the presence of the methyl groups (CH3) in the acetate ions, which are nonpolar. This dual nature makes a clear classification difficult.
According to Dr. Anya Sharma, a lead chemist at National Chemical Research Institute, "The behavior of Acetate of Lime is not straightforward. While the ionic character promotes polarity, the organic acetate group introduces nonpolar characteristics."
Solubility Studies
Solubility tests are providing some clues. Acetate of Lime demonstrates good solubility in water, a polar solvent, suggesting a predominantly polar nature.
However, it exhibits limited solubility in nonpolar solvents like hexane, further complicating the determination.
Dipole Moment Analysis
Advanced analytical techniques, like dipole moment analysis, are being employed. Initial findings suggest a measurable dipole moment, indicating a polar characteristic.
However, the magnitude of the dipole moment is relatively small compared to highly polar compounds, leaving room for debate.
Implications Across Industries
The pharmaceutical industry is heavily impacted. Accurate polarity data is crucial for drug formulation and delivery.
In the textile industry, the correct polarity assessment affects dyeing and finishing processes. Misclassification can result in uneven color distribution and compromised fabric quality.
The food industry relies on precise knowledge of its properties to ensure proper ingredient mixing and stability in various food products. This is particularly relevant in processes where Acetate of Lime is used as a preservative or buffer.
The Road Ahead: Calls for Standardized Testing
The scientific community is calling for standardized testing protocols. The need for a consistent and reliable method to determine the polarity of Acetate of Lime is urgent.
The International Union of Pure and Applied Chemistry (IUPAC) is considering developing a specific guideline for classifying compounds with both polar and nonpolar characteristics.
Efforts are underway at the American Chemical Society to organize a collaborative study involving multiple laboratories to generate definitive data. These multi-lab tests are crucial for ensuring the reliability and reproducibility of the results.
Conclusion: Awaiting Definitive Results
The question of whether Acetate of Lime is polar or nonpolar remains unresolved. Ongoing research and standardization efforts are critical to provide clarity.
Until a definitive answer emerges, industries are urged to proceed with caution and thoroughly validate their processes using Acetate of Lime. The consequences of misclassification could be significant and costly.