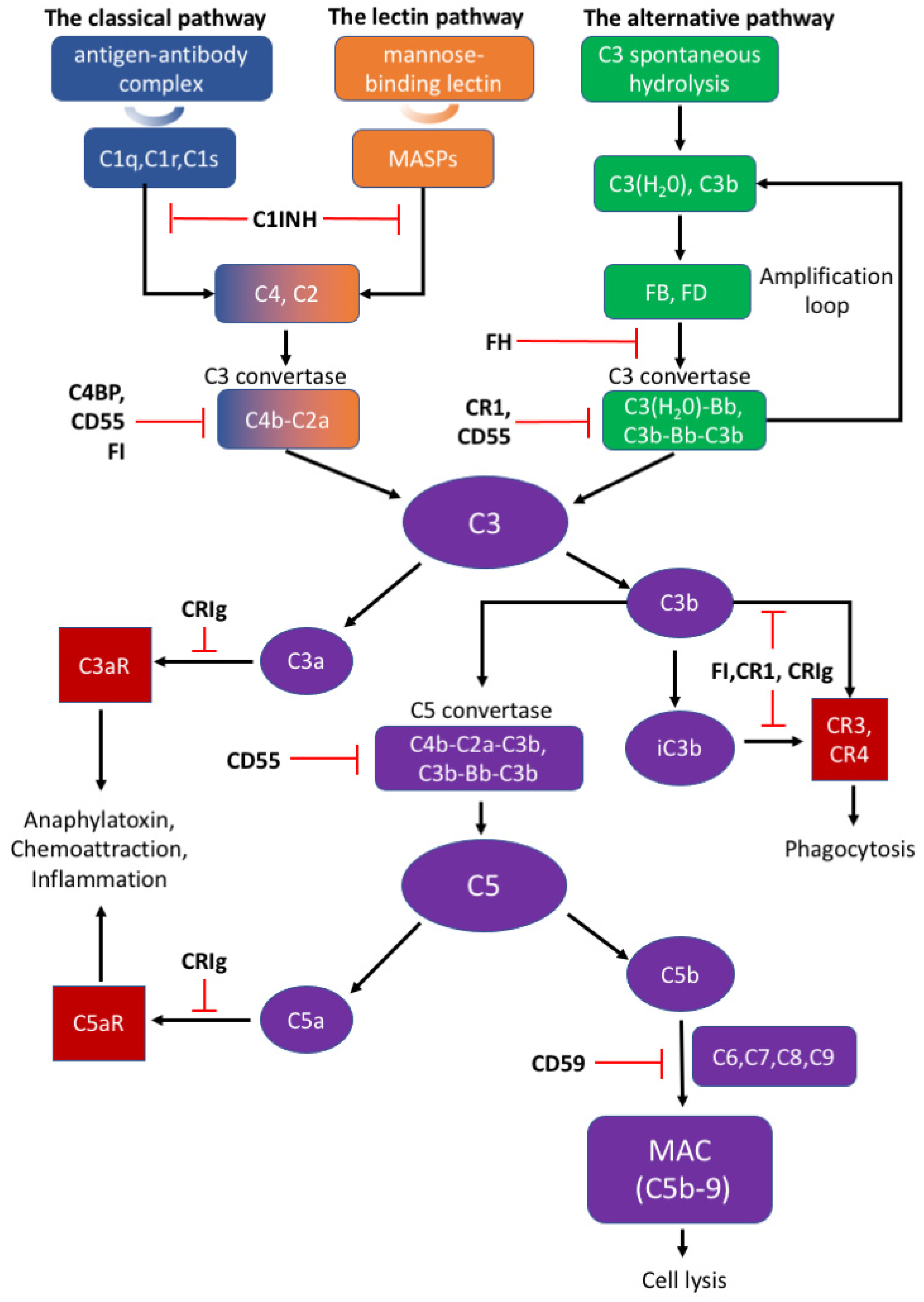
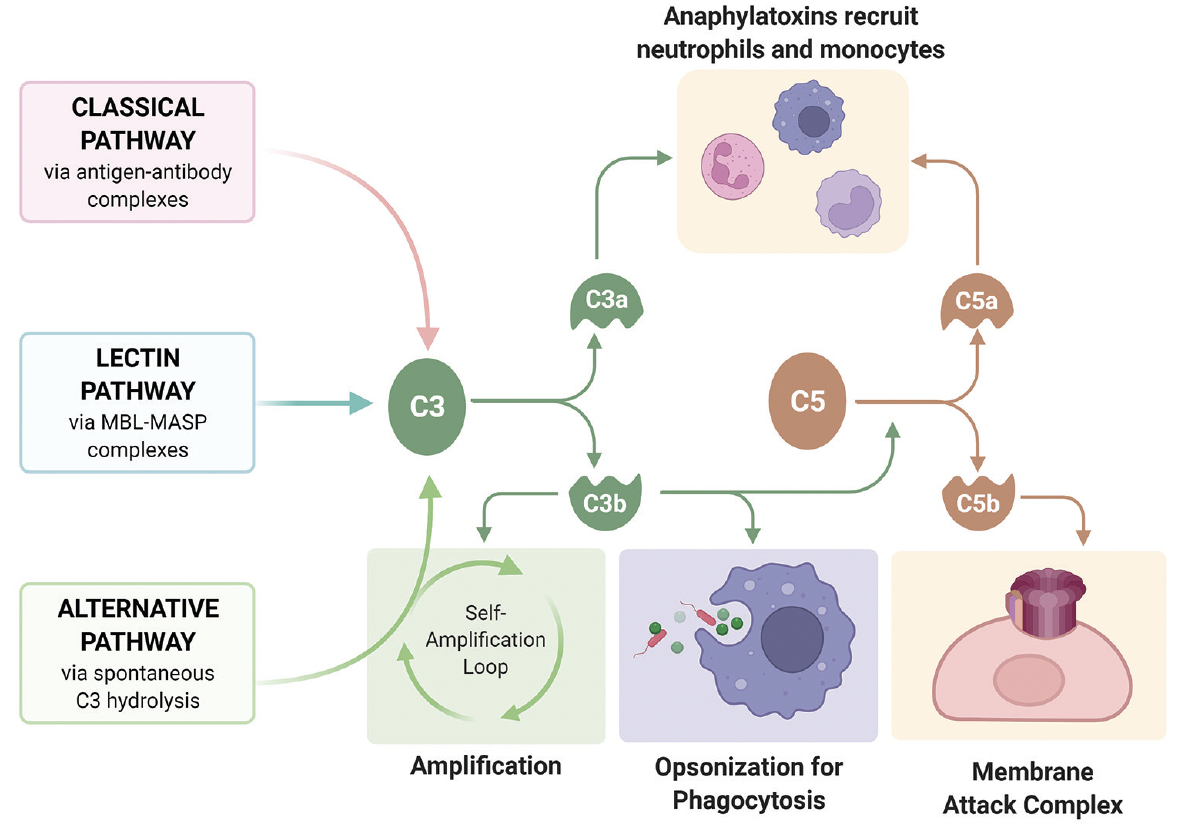
All Three Pathways For Complement Activation

The human body possesses a sophisticated defense system, the complement system, crucial for immune surveillance and protection against pathogens. This complex network, consisting of over 30 proteins, acts as a rapid-response mechanism to identify and eliminate threats. Understanding the intricate workings of its three activation pathways – classical, alternative, and lectin – is paramount for comprehending immunity and developing targeted therapies for various diseases.
The complement system's importance extends far beyond basic immunity. Its dysregulation is implicated in a wide range of disorders, including autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus, as well as neurodegenerative conditions and cancer. A deeper comprehension of these pathways offers hope for innovative treatments that can modulate complement activity and improve patient outcomes.
The Classical Pathway: Antibody-Mediated Activation
The classical pathway is initiated when the C1 complex, comprised of C1q, C1r, and C1s proteins, binds to antibodies (IgG or IgM) that are already attached to an antigen, such as a bacterium or a virus. This antibody-antigen complex serves as the trigger for the pathway. This binding activates C1r, which in turn activates C1s.
Activated C1s then cleaves C4 into C4a and C4b. C4b binds to the target cell's surface. Subsequently, C1s cleaves C2 into C2a and C2b. C2a binds to C4b, forming the C4b2a complex, also known as the classical pathway C3 convertase. This convertase cleaves C3, initiating the downstream effects of complement activation.
The Alternative Pathway: Spontaneous Activation
Unlike the antibody-dependent classical pathway, the alternative pathway is continuously and spontaneously activated at a low level. This "tick-over" is due to the spontaneous hydrolysis of C3 in the plasma, forming C3(H2O). This pathway is crucial for early detection of pathogens before antibodies are produced.
C3(H2O) binds to Factor B, which is then cleaved by Factor D into Ba and Bb. The Bb fragment remains bound to C3(H2O), forming the C3(H2O)Bb complex, a fluid-phase C3 convertase. This convertase cleaves more C3, creating C3a and C3b, amplifying the cascade. The C3b fragment can then bind to the pathogen surface.
The stability of the alternative pathway is regulated by Properdin (Factor P), which binds to and stabilizes the C3bBb complex on the pathogen surface. Factors H and I act as inhibitors of the alternative pathway, preventing excessive activation. They facilitate the degradation of C3b, preventing the formation of the C3 convertase on host cells.
The Lectin Pathway: Sugar Recognition
The lectin pathway is activated when mannose-binding lectin (MBL) or ficolins, pattern recognition receptors (PRRs), bind to specific carbohydrate structures on the surface of pathogens. These carbohydrates, such as mannose, are commonly found on bacteria, fungi, and viruses, but are rare on human cells. This pathway bridges innate and adaptive immunity.
Upon binding to carbohydrates, MBL or ficolins activate MBL-associated serine proteases (MASPs). These proteases, primarily MASP-1 and MASP-2, are structurally and functionally similar to C1r and C1s of the classical pathway. Activated MASP-2 cleaves C4 and C2, similar to the classical pathway. Resulting in the formation of the C4b2a complex, the C3 convertase.
The Common Outcome: Pathogen Elimination
Despite their different activation mechanisms, all three pathways converge at the formation of the C3 convertase. This enzyme cleaves C3 into C3a and C3b, initiating a cascade of events that leads to pathogen elimination. C3b opsonizes pathogens, marking them for phagocytosis by immune cells like macrophages and neutrophils.
Furthermore, C3b binds to the C3 convertase, forming the C5 convertase. This enzyme cleaves C5 into C5a and C5b. C5a is a potent anaphylatoxin, attracting immune cells to the site of infection and promoting inflammation. C5b initiates the formation of the membrane attack complex (MAC).
The MAC, composed of C5b, C6, C7, C8, and multiple C9 molecules, inserts itself into the pathogen's cell membrane, forming pores that disrupt the cell's integrity and lead to lysis. This is a direct and powerful mechanism for killing pathogens.
Therapeutic Implications and Future Directions
Research into the complement system is rapidly advancing, with significant implications for therapeutic development. Complement inhibitors, such as eculizumab, are already used to treat certain rare diseases like paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS), which involve uncontrolled complement activation.
Scientists are exploring new strategies to selectively target specific complement pathways or components, aiming to minimize off-target effects and maximize therapeutic efficacy. This includes developing novel antibodies, small molecule inhibitors, and gene therapies to modulate complement activity in various diseases.
A deeper understanding of the intricate interplay between the three complement pathways and their roles in both health and disease holds immense promise for the development of more effective and targeted therapies. These advancements could revolutionize the treatment of autoimmune disorders, infectious diseases, and other conditions where complement dysregulation plays a significant role.






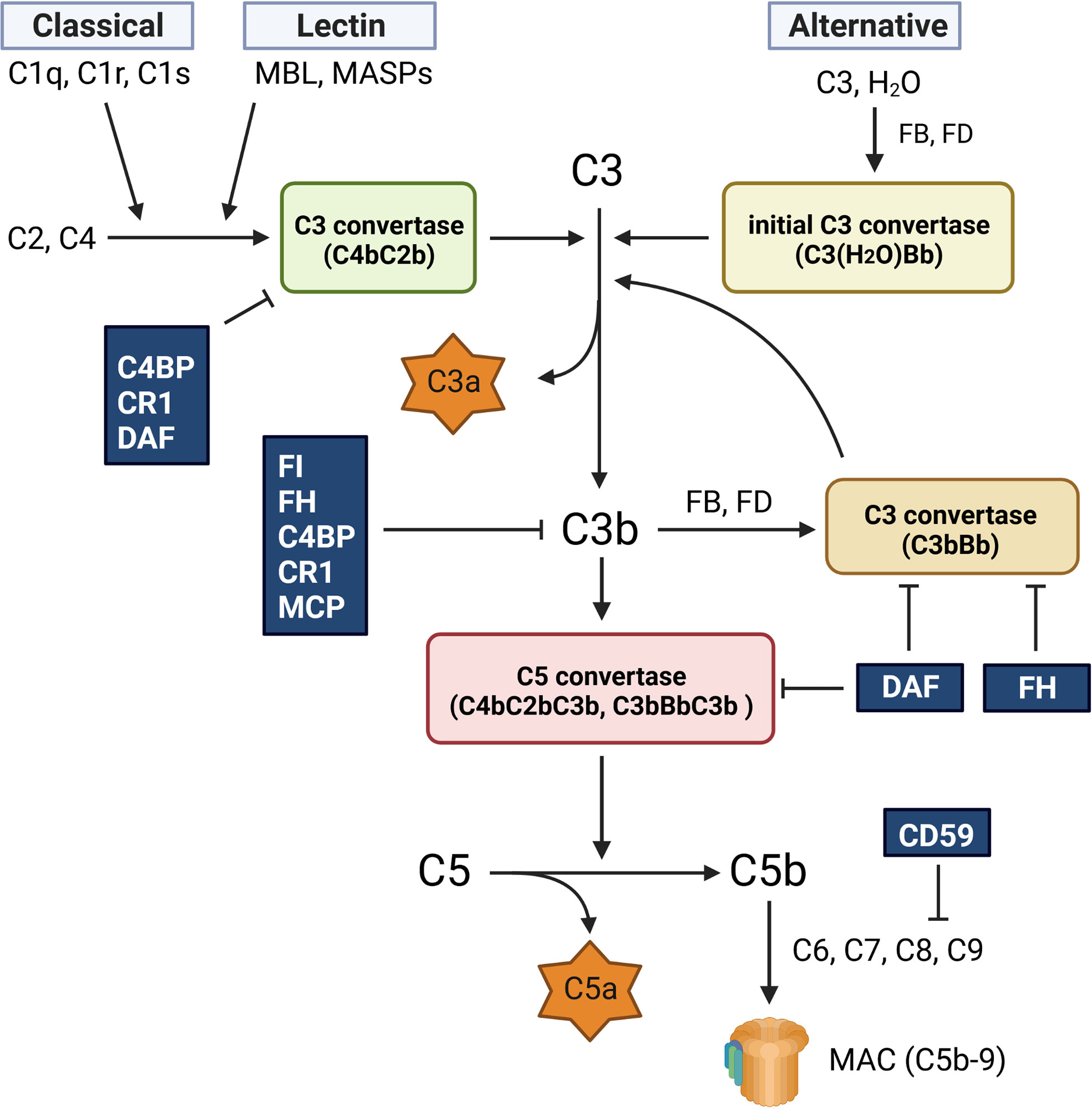
![All Three Pathways For Complement Activation Complement activation pathways [42, 48, 52]. The three complement](https://www.researchgate.net/publication/357165895/figure/fig1/AS:1102707301593088@1639917316092/Complement-activation-pathways-42-48-52-The-three-complement-activation-pathways-the.png)
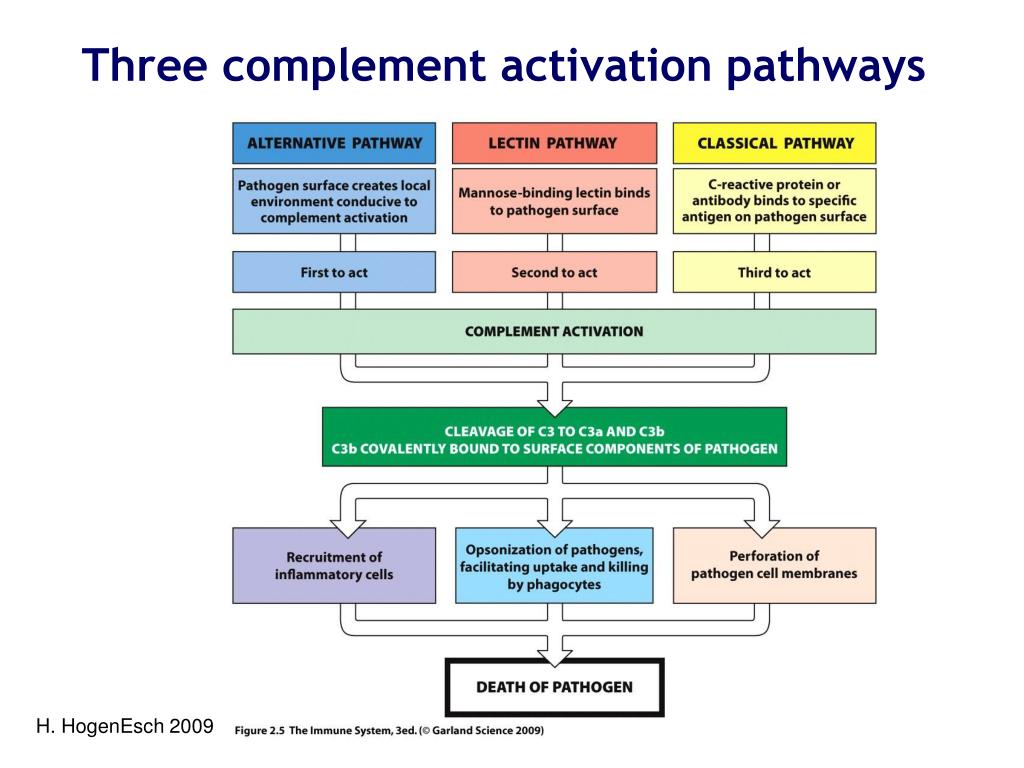
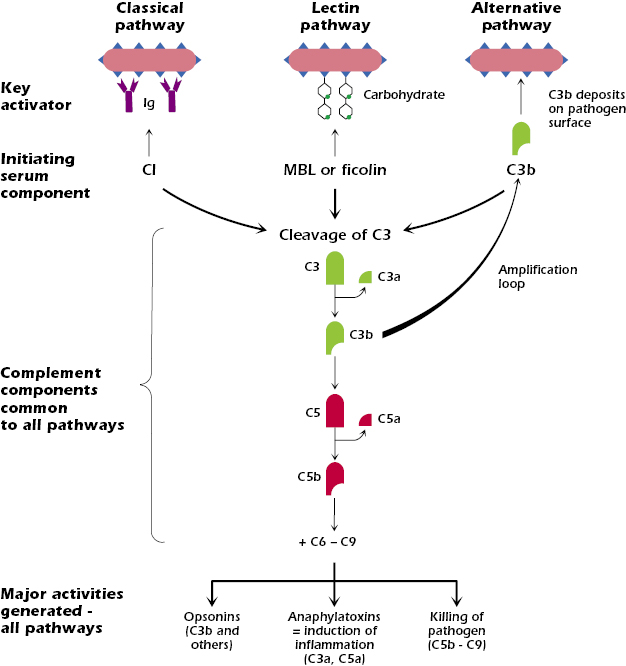





70167-8/asset/1c8b2064-f6e3-4577-ab7b-561590fb0e59/main.assets/gr1_lrg.jpg)