Aortic Stent Grafts Pipeline Products Market

The global aortic stent grafts pipeline products market is experiencing dynamic growth, fueled by advancements in medical technology and an aging population increasingly susceptible to aortic aneurysms and other vascular diseases.
This expanding market encompasses a range of devices in various stages of development, from preclinical research to commercial launch, offering hope for improved treatment options and patient outcomes.
The anticipated impact of these pipeline products on healthcare systems and patient lives is substantial, promising less invasive procedures, faster recovery times, and enhanced long-term results.
Understanding the Aortic Stent Grafts Pipeline
The "pipeline" refers to the different stages of development that medical devices, like aortic stent grafts, undergo before they are available for widespread clinical use.
This process typically includes preclinical research, clinical trials (Phase I, II, and III), regulatory review and approval, and finally, market launch.
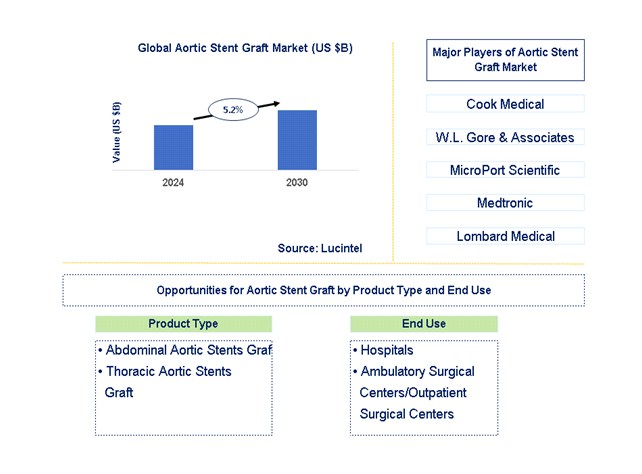
The current aortic stent grafts pipeline is characterized by a diverse array of innovations targeting various segments of the aorta, including the thoracic and abdominal regions.
Key Players and Innovations
Several major players are actively involved in developing novel aortic stent grafts.
Medtronic, Cook Medical, W. L. Gore & Associates, and Terumo Aortic are among the leading companies investing heavily in research and development.
Their efforts are focused on creating grafts with improved durability, enhanced conformability to complex anatomies, and reduced rates of complications such as endoleaks.
One notable trend is the development of fenestrated and branched stent grafts, designed to preserve blood flow to critical branch vessels arising from the aorta, such as the renal and mesenteric arteries.
These specialized grafts require meticulous planning and execution, but they offer a less invasive alternative to open surgical repair for complex aortic aneurysms.
Furthermore, research is underway to develop bioabsorbable stent grafts, which would gradually dissolve over time after providing initial support to the damaged aorta.
This technology holds the potential to eliminate the long-term risks associated with permanent implants, such as infection and stent migration.
Clinical Trials and Regulatory Landscape
Clinical trials play a crucial role in evaluating the safety and efficacy of new aortic stent grafts.
These trials involve rigorous testing on human subjects, following strict protocols and ethical guidelines.
The results of clinical trials are then submitted to regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), for review and approval.
Obtaining regulatory approval is a critical step in bringing a new aortic stent graft to market.
The approval process ensures that the device meets stringent safety and performance standards.
The timeline for regulatory approval can vary depending on the complexity of the device and the specific requirements of each regulatory agency.
Impact and Future Outlook
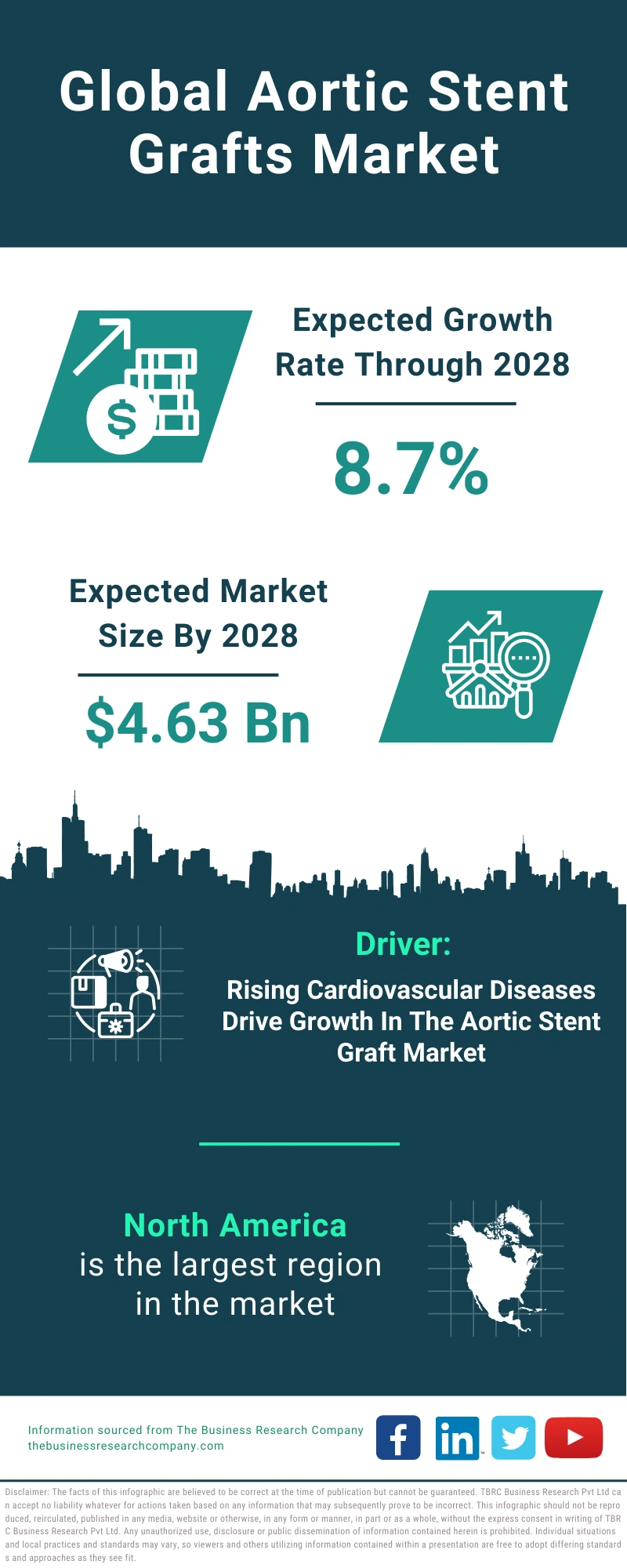
The aortic stent grafts pipeline products market holds significant promise for improving the treatment of aortic diseases.
By offering less invasive alternatives to traditional open surgery, these devices can reduce patient morbidity, shorten hospital stays, and improve overall quality of life.
The growing prevalence of aortic aneurysms, driven by factors such as aging and lifestyle choices, is expected to further fuel the demand for aortic stent grafts.
However, challenges remain in ensuring equitable access to these advanced technologies and in addressing the high cost of development and manufacturing.
Collaboration between researchers, clinicians, and industry partners is essential to overcome these challenges and to accelerate the development of innovative aortic stent grafts.
The future of the aortic stent grafts market looks promising, with ongoing research and development efforts focused on creating even more effective, durable, and patient-friendly devices.
The convergence of technological advancements, clinical expertise, and regulatory support will pave the way for a new era in the treatment of aortic diseases.
This will ultimately benefit patients worldwide by providing them with access to life-saving treatments that are less invasive, more effective, and more affordable.