Approximately How Many Elements Are There

The periodic table, the cornerstone of chemistry, is now considered to contain 118 elements. This count was officially finalized in 2016, marking the completion of the seventh row.
For those needing a quick reference: The total number of confirmed elements stands at 118, each with unique properties and atomic structures.
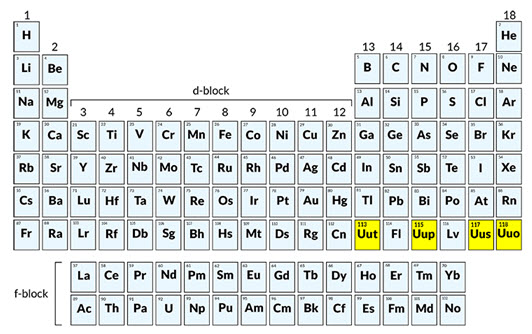
The Current Count: 118 Elements
As of now, the generally accepted number of elements is 118. These elements are organized in the periodic table based on their atomic number, electron configuration, and recurring chemical properties.
Discovery and Synthesis
The discovery of elements has been a centuries-long endeavor, with some elements known since antiquity. Many of the heavier elements, those beyond uranium, are synthetically produced in laboratories.
These man-made elements are created through nuclear reactions. Scientists use particle accelerators to collide atomic nuclei, fusing them to create new, heavier elements that do not naturally occur.
Natural vs. Synthetic Elements
Of the 118 elements, 94 occur naturally on Earth. The remaining 24 are synthetic, meaning they were created in laboratories.
The last naturally occurring element to be discovered was francium (atomic number 87) in 1939. Synthetic elements often have short half-lives and decay rapidly.
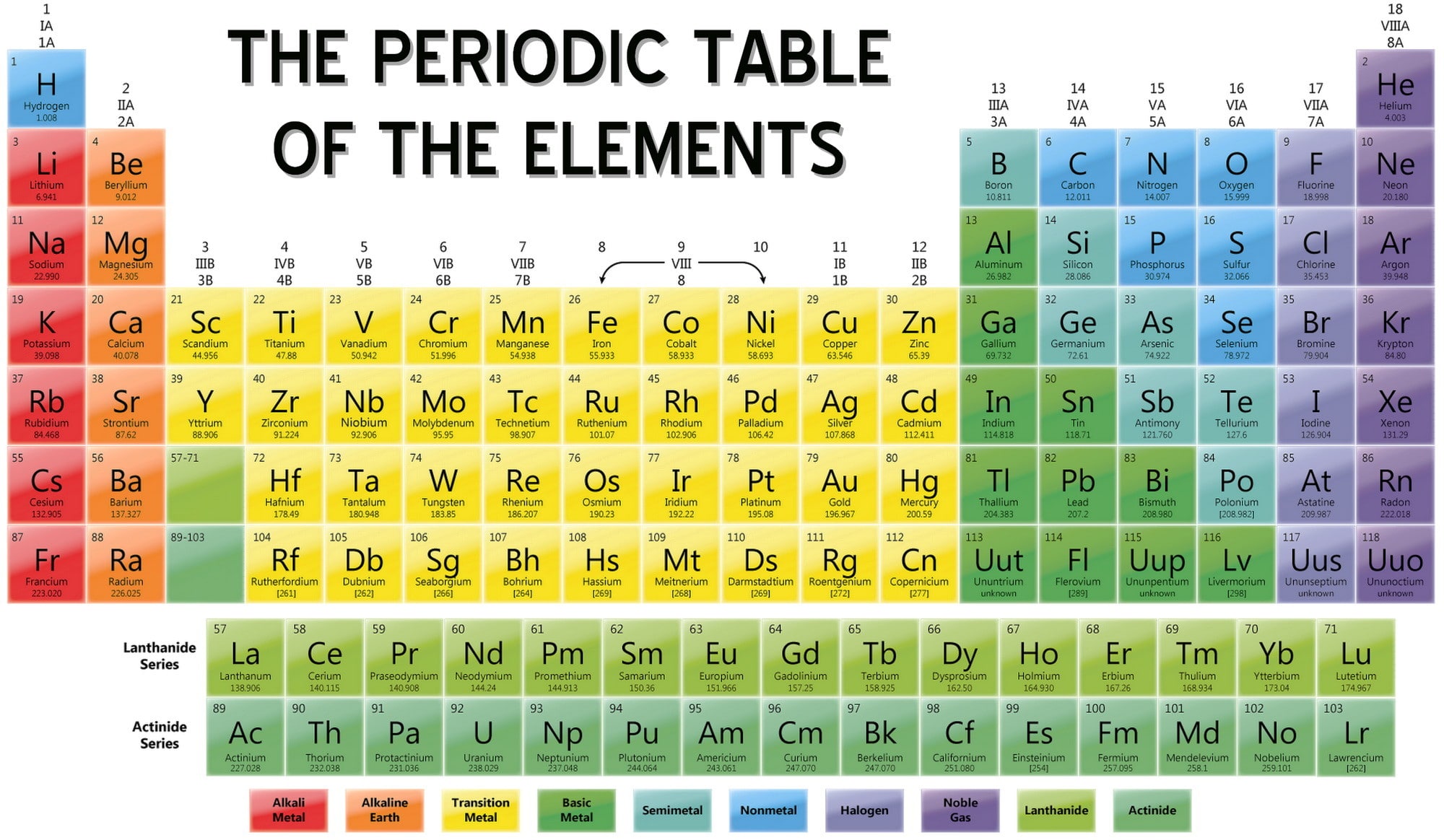
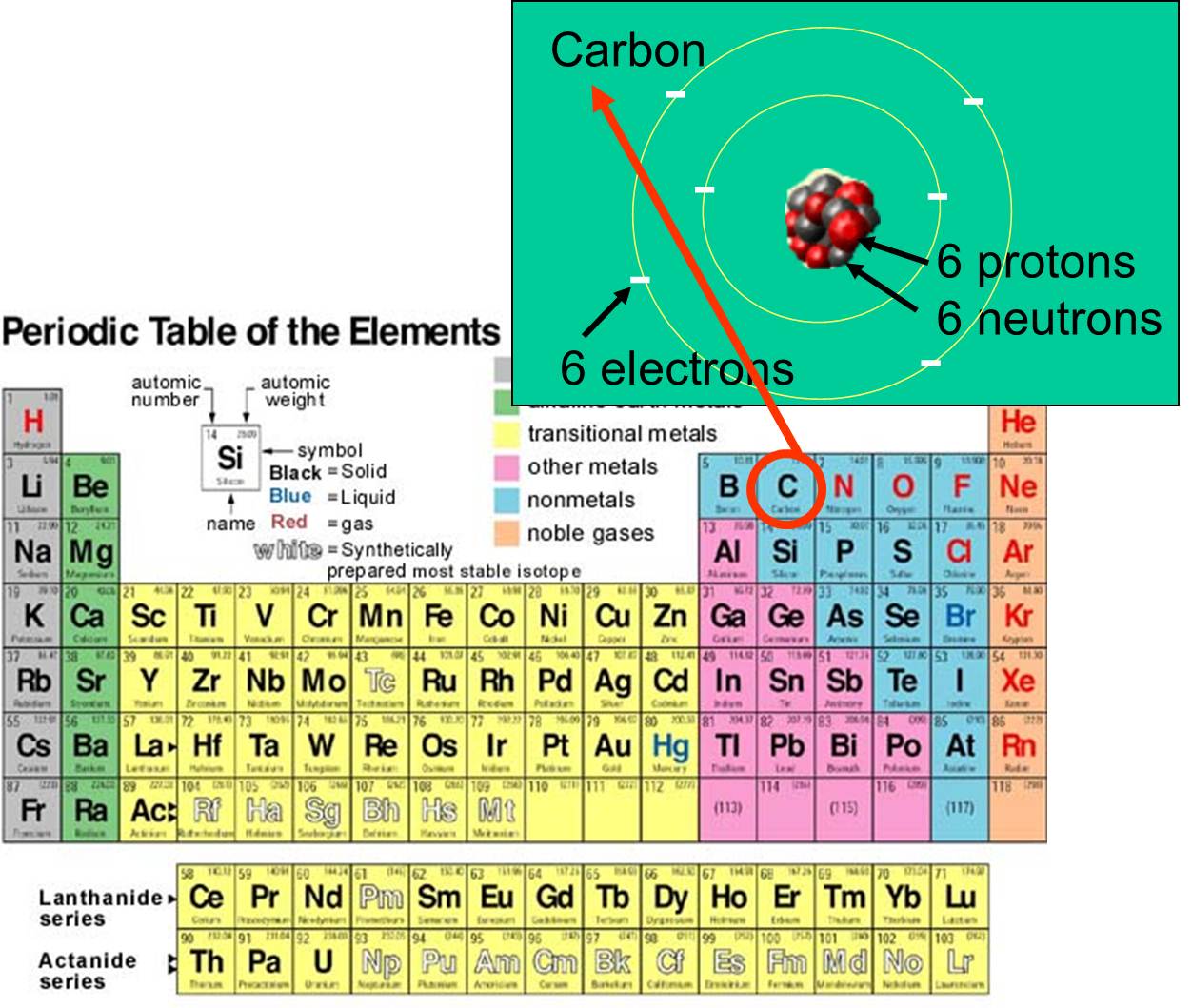
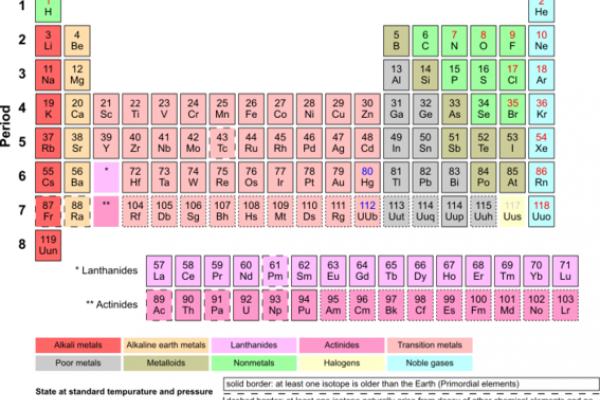
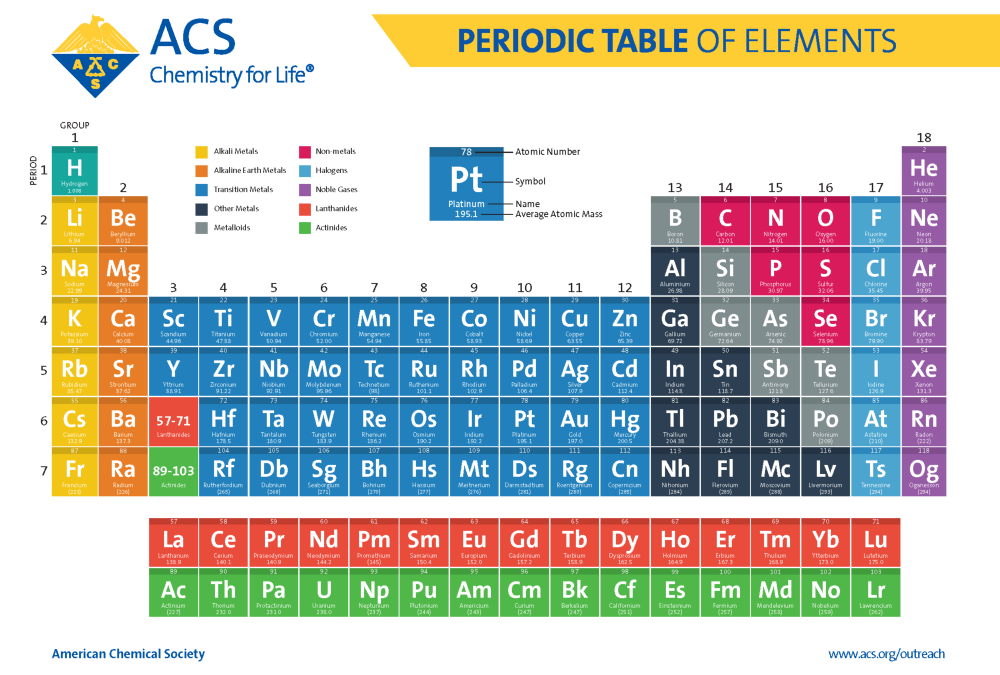
The Periodic Table
The periodic table, developed by Dmitri Mendeleev in the 19th century, is a tabular arrangement of the chemical elements. It's organized by atomic number, electron configuration, and recurring chemical properties.
The table's rows are called periods, and the columns are called groups or families. Elements in the same group share similar chemical behaviors. Each cell in the table contains symbol of the element and its atomic number.
Filling the Seventh Row
The seventh row of the periodic table was completed in 2016 with the synthesis of elements 113, 115, 117, and 118. These elements were nihonium (Nh), moscovium (Mc), tennessine (Ts), and oganesson (Og), respectively.
These discoveries were officially recognized by the International Union of Pure and Applied Chemistry (IUPAC). IUPAC is the globally recognized authority for chemical nomenclature and terminology.
How We Know: Confirmation Process
The discovery of a new element requires rigorous verification and confirmation. Research teams must provide substantial evidence to support their claim.
This evidence typically includes detailed data on the element's atomic properties and decay patterns. The findings are then scrutinized by IUPAC before official recognition. This confirmation process involves multiple independent verifications by other labs.
Naming Conventions
Once an element is confirmed, the discoverers have the right to propose a name and symbol for it. The names are often derived from places, scientists, or mythological figures.
IUPAC reviews the proposed name to ensure it aligns with established naming conventions. The final name must be approved by IUPAC before it becomes official.
Looking Ahead: The Future of Element Discovery
Scientists are currently exploring the possibility of synthesizing elements beyond oganesson (element 118). These hypothetical elements would begin the eighth row of the periodic table.
The search for these elements poses significant challenges. Superheavy elements are extremely unstable and have very short half-lives, making them difficult to detect and study.
Challenges and Limitations
Synthesizing new elements requires advanced technology and significant resources. Particle accelerators and specialized detectors are essential for these experiments.
Theoretical calculations suggest that there may be an "island of stability" where superheavy elements could have longer half-lives. However, reaching this island remains a major scientific goal.
The extreme instability and short life-spans of superheavy elements complicate their study.