Ascend Triamcinolone Acetonide Cream Usp

URGENT: Ascend Laboratories is initiating a nationwide voluntary recall of one lot of Triamcinolone Acetonide Cream USP, 0.1%, due to a potential lack of tamper evidence. This recall, announced immediately, impacts a specific lot distributed across the United States.
The recall stems from concerns that the crimp on the tube of the affected lot might not adequately prevent tampering. This compromises the integrity of the medication, potentially posing a risk to consumers.
Recall Details
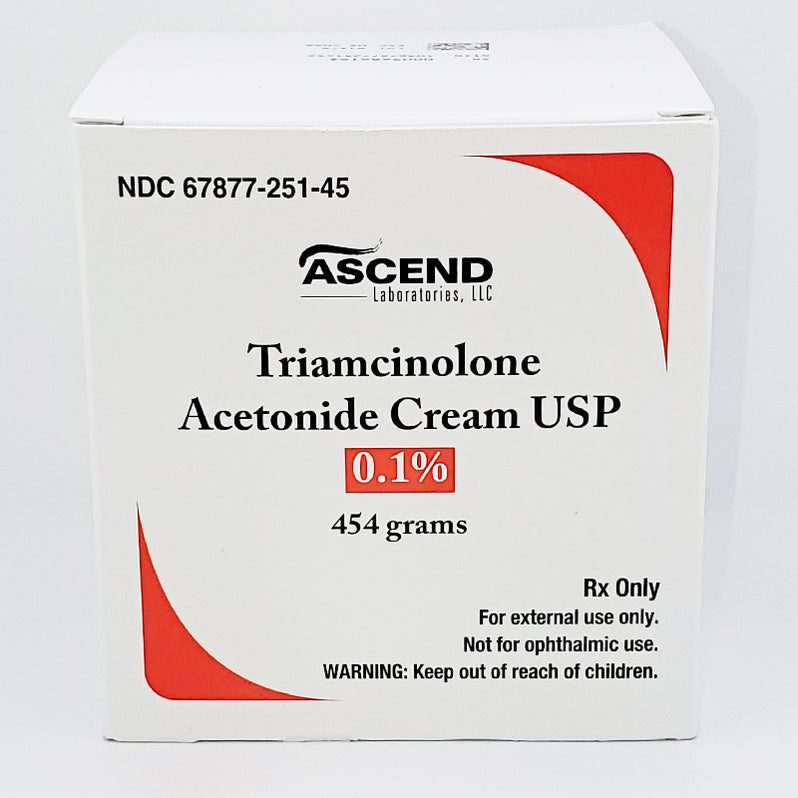
What: Ascend Laboratories is recalling one lot of Triamcinolone Acetonide Cream USP, 0.1%.
Lot Number: Affected lot is identified as BT028A, with an expiration date of December 2025.
Strength: The recalled product is Triamcinolone Acetonide Cream USP, 0.1%.
Packaging: The cream is packaged in a tube and carton.
Who: The recall impacts patients, pharmacies, and healthcare providers who may have received the affected lot.
Where: The product was distributed nationwide throughout the United States.
When: Ascend Laboratories initiated the voluntary recall immediately following internal identification of the issue.
Potential Risks
The primary risk associated with the potential lack of tamper evidence is the possibility that the product has been compromised. This could include adulteration or contamination, rendering the cream ineffective or even harmful.
Patients using potentially tampered product might not receive the intended therapeutic effect. Furthermore, contaminated product could lead to local skin reactions, infections, or other adverse health consequences.
Ascend Laboratories' Response
Ascend Laboratories is notifying distributors and customers by recall letter. The letter outlines instructions for returning the recalled product.
They are also arranging for the return of all recalled products to Ascend Laboratories.
Ascend Laboratories is committed to patient safety and is taking this action proactively.
Consumer Action
Consumers who have the affected lot of Triamcinolone Acetonide Cream USP, 0.1% (Lot BT028A, Exp. 12/2025) should immediately stop using the product.
Return the product to the place of purchase or contact Ascend Laboratories directly for instructions on how to return the affected lot.
Contact your physician or healthcare provider if you have experienced any problems that may be related to using this drug product.
Reporting Adverse Reactions
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program.
Reports can be submitted online, by regular mail, or by fax.
Visit www.fda.gov/medwatch/report.htm for more information.
Contact Information
For questions regarding this recall, consumers can contact Ascend Laboratories at the phone number or email address provided in the recall letter.
Specific contact details can be found on the Ascend Laboratories website.
Next Steps
Ascend Laboratories is working with the FDA to ensure the effectiveness of the recall and to prevent future occurrences.
The company is investigating the root cause of the crimp issue to implement corrective actions.
The FDA will continue to monitor the recall and provide updates as necessary.