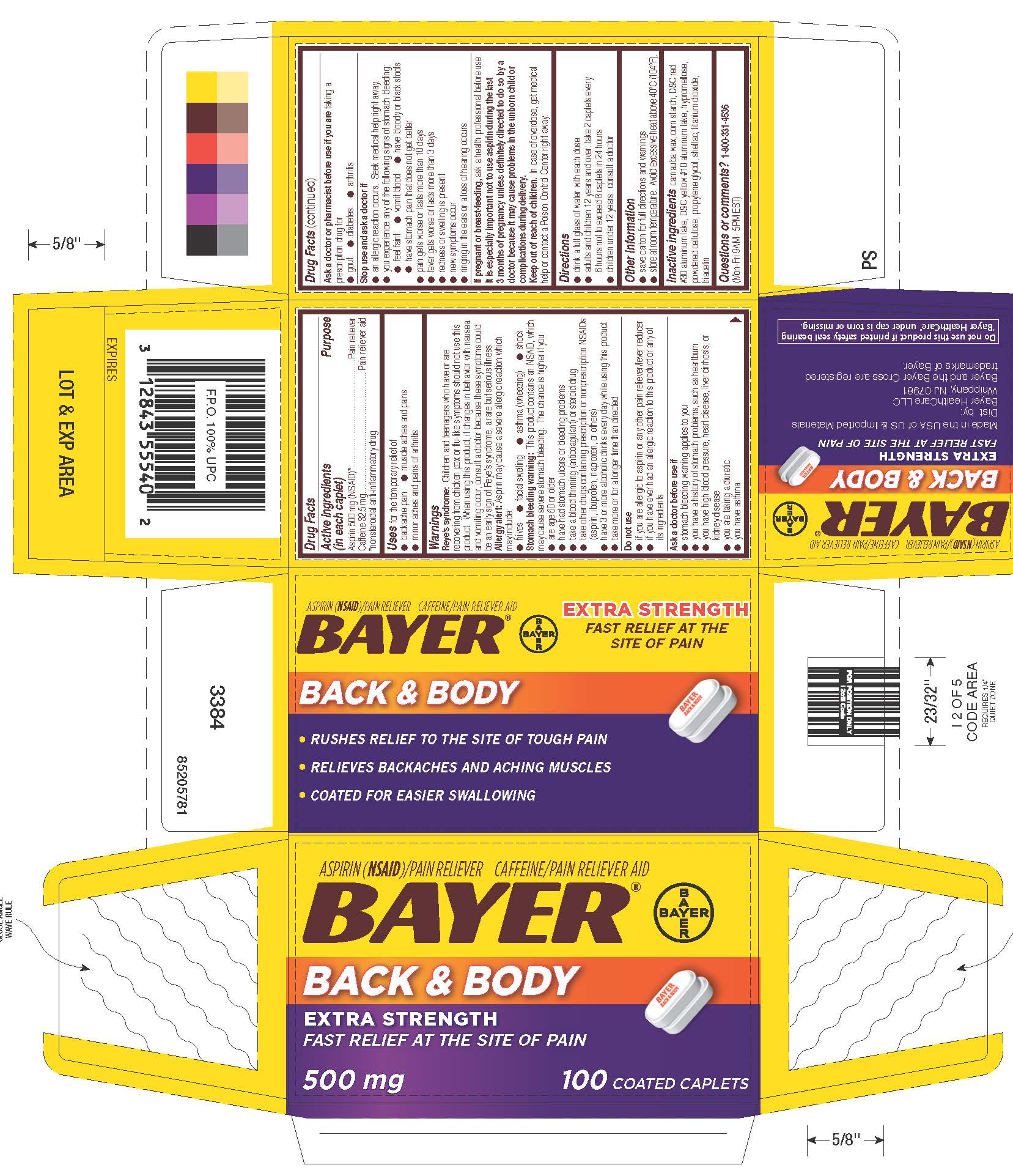
Bayer Back And Body Side Effects

The over-the-counter pain reliever, Bayer Back & Body, marketed by Bayer AG, has long been a staple in medicine cabinets for those seeking relief from aches and pains. However, mounting concerns are emerging regarding the potential side effects associated with its active ingredients, particularly the risk of gastrointestinal bleeding and cardiovascular complications.
These concerns, fueled by independent research and increasing adverse event reports, have prompted renewed scrutiny of the drug's safety profile and the adequacy of warnings provided to consumers.
The situation has prompted questions about the balance between the drug's perceived benefits and the potential risks, forcing both Bayer and regulatory agencies to re-evaluate its place in the consumer healthcare landscape.
Understanding the Controversy Surrounding Bayer Back & Body
At the heart of the issue lies the active ingredient: usually either aspirin or a nonsteroidal anti-inflammatory drug (NSAID) like ibuprofen or naproxen. These drugs work by inhibiting the production of prostaglandins, chemicals in the body that contribute to inflammation and pain.
While effective in alleviating discomfort, this mechanism also interferes with the body's natural protective mechanisms, particularly in the gastrointestinal tract and cardiovascular system.
This article will delve into the specific side effects associated with Bayer Back & Body, the scientific evidence supporting these concerns, and the responses from Bayer and regulatory bodies.
Gastrointestinal Risks: A Clear and Present Danger
One of the most well-documented side effects of NSAIDs, including those found in Bayer Back & Body, is the increased risk of gastrointestinal bleeding.
By inhibiting prostaglandin production, these drugs can reduce the stomach's ability to produce protective mucus, making it more vulnerable to damage from stomach acid.
Studies have consistently shown a correlation between NSAID use and an increased risk of ulcers, gastritis, and potentially life-threatening gastrointestinal bleeds. Individuals with a history of ulcers, older adults, and those taking other medications that thin the blood are at significantly higher risk.
"The risk of gastrointestinal bleeding is a well-established side effect of NSAIDs. Patients should always be aware of the potential signs and symptoms and seek medical attention if they experience them,"said Dr. Emily Carter, a gastroenterologist at the University of California, San Francisco.
Cardiovascular Concerns: A Growing Body of Evidence
In recent years, concerns have also grown regarding the potential cardiovascular risks associated with NSAID use, including Bayer Back & Body.
Some studies have suggested an increased risk of heart attack, stroke, and high blood pressure, particularly with long-term or high-dose use.
The exact mechanism by which NSAIDs may contribute to these cardiovascular events is still under investigation, but it is believed to involve their effects on blood clotting and blood vessel constriction.
The FDA has issued warnings about the potential cardiovascular risks associated with NSAIDs, advising that they should be used at the lowest effective dose for the shortest duration necessary. Patients with pre-existing heart conditions should exercise particular caution and consult with their doctor before taking Bayer Back & Body.
Bayer's Response and Regulatory Oversight
Bayer AG, the manufacturer of Bayer Back & Body, maintains that its products are safe and effective when used as directed. The company emphasizes the importance of following dosage instructions and reading the product label carefully.
In a statement released to the public, Bayer stated, "Patient safety is our top priority. We continuously monitor the safety of our products and work closely with regulatory agencies to ensure that our products are used safely and effectively."
However, critics argue that the warnings provided on Bayer Back & Body packaging are not prominent enough and do not adequately convey the potential risks, especially for vulnerable populations.
The FDA is responsible for regulating over-the-counter drugs like Bayer Back & Body. The agency has the authority to require manufacturers to update their labeling, issue safety alerts, and even remove products from the market if they are deemed unsafe.
The FDA is currently reviewing the safety data associated with NSAIDs and may consider further regulatory action if necessary.
The Consumer Perspective: Balancing Relief and Risk
For many consumers, Bayer Back & Body provides effective relief from pain and discomfort, allowing them to maintain an active lifestyle. However, the potential side effects raise important questions about risk-benefit assessment.
Consumers need to be fully informed about the potential risks associated with Bayer Back & Body so they can make informed decisions about their healthcare. This includes understanding the signs and symptoms of gastrointestinal bleeding and cardiovascular events and knowing when to seek medical attention.
Furthermore, exploring alternative pain management strategies, such as physical therapy, exercise, and other non-pharmacological approaches, may be beneficial for some individuals.
The Road Ahead: Ensuring Patient Safety
The ongoing debate surrounding Bayer Back & Body highlights the importance of continuous monitoring of drug safety and the need for clear and transparent communication between manufacturers, regulatory agencies, and consumers.
Moving forward, greater emphasis should be placed on patient education, ensuring that individuals are fully aware of the potential risks and benefits of Bayer Back & Body before using it.
Further research is also needed to better understand the mechanisms by which NSAIDs may contribute to cardiovascular events and to identify individuals who are at higher risk. Ultimately, the goal is to strike a balance between providing effective pain relief and protecting patient safety.