Burning Of Wood Chemical Or Physical Change

The acrid smell of smoke and the crackling sound of flames are familiar signs of burning wood. But beneath this seemingly simple act lies a complex interplay of chemical and physical transformations, a process vital to understanding everything from campfire safety to large-scale wildfires.
Understanding whether burning wood constitutes a chemical or physical change (or both) is crucial for diverse fields, including forestry management, environmental science, and even everyday activities like cooking. The debate often centers around the nature of the changes occurring to the wood's composition at a molecular level.
The Science of Combustion
At its core, burning wood, or combustion, is a rapid chemical process that requires three elements: fuel (the wood), an oxidant (usually oxygen), and an ignition source (heat). When wood is heated to its ignition temperature, it undergoes a series of chemical reactions.
This temperature typically ranges from 300-600°C. These reactions release heat and light, sustaining the combustion process.
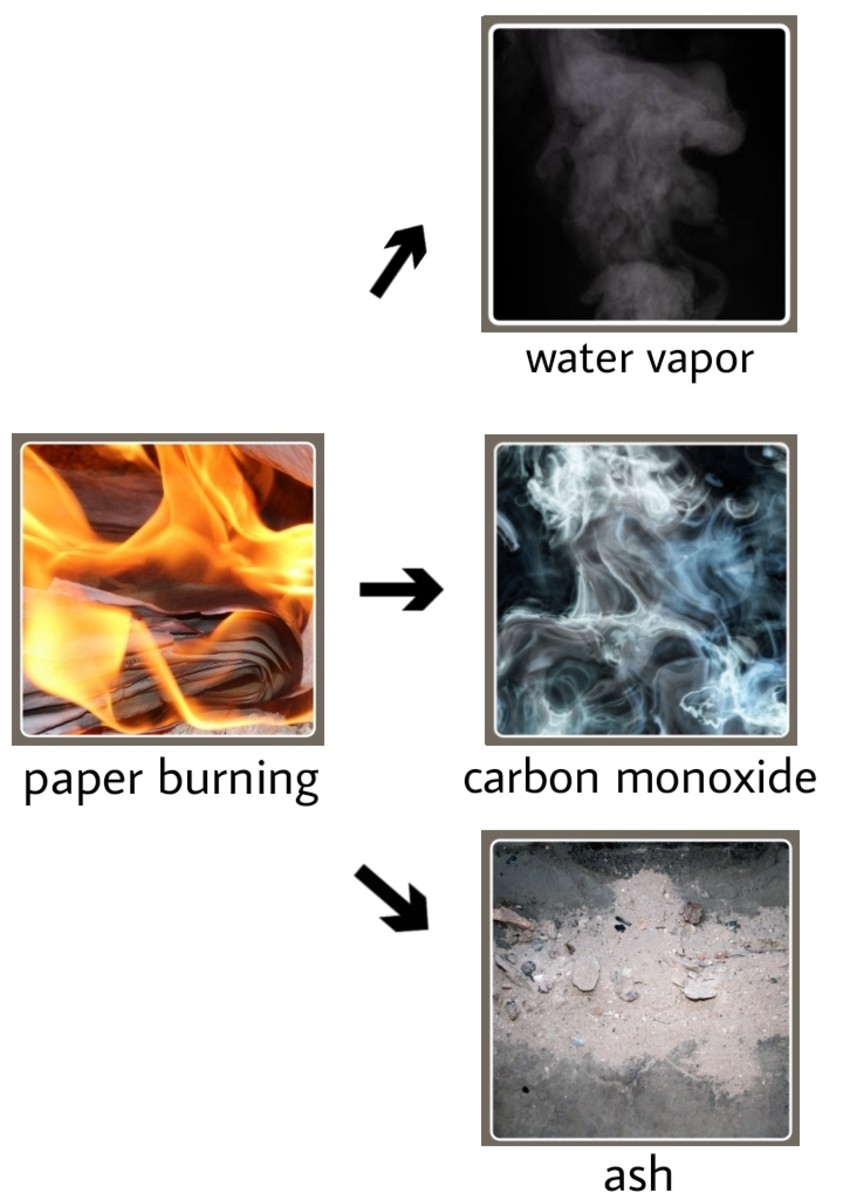
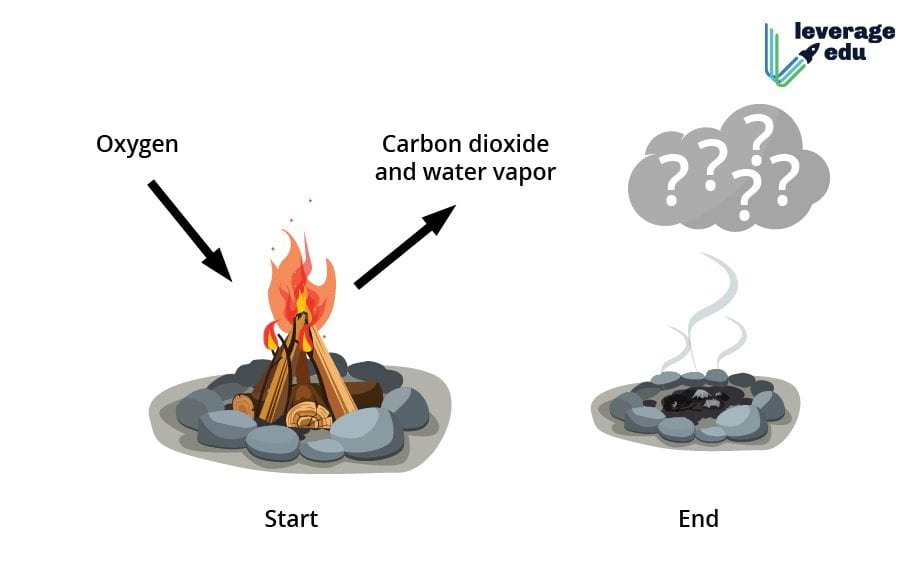
During combustion, the complex organic compounds in wood, primarily cellulose, hemicellulose, and lignin, react with oxygen. This reaction produces new substances, most notably carbon dioxide (CO2) and water (H2O), along with ash and various other byproducts.
Key Detail: The transformation of wood into CO2 and H2O represents a change in the chemical composition of the substance, confirming it as a chemical change.
The Chemical Change Argument
The strongest argument for burning wood being a chemical change lies in the formation of entirely new substances. The original wood is no longer present after burning, instead, replaced by ash, gases, and other compounds.
For example, cellulose, a long-chain polymer of glucose, is broken down into simpler molecules and ultimately oxidized into CO2 and H2O. This chemical decomposition is irreversible under normal conditions.
“The rearrangement of atoms and the breaking and forming of chemical bonds are definitive characteristics of a chemical change,” explains Dr. Emily Carter, a professor of chemical engineering at California Institute of Technology, in a recent interview.
Physical Changes Involved
While combustion is primarily a chemical process, physical changes also occur during the burning of wood. These include changes in state and alterations in physical form.
As wood heats up, water molecules within its structure evaporate, changing from a liquid to a gas. This phase transition is a classic example of a physical change.
Additionally, wood shrinks and cracks due to the heat, its structure breaking down into smaller pieces. However, these changes don't alter the chemical composition of the wood until combustion occurs.
Quote: “The wood is initially dried by heat, and then pyrolysis breaks down the wood into volatile gases and charcoal, which then combusts,” states a report by the National Fire Protection Association (NFPA). This quote highlights the step-by-step process involving both physical and chemical change.
Significance and Impact
Understanding the chemical and physical processes involved in burning wood has significant implications. It affects forest fire prevention, the design of efficient wood-burning stoves, and understanding the role of biomass in energy production.
Controlling wildfires requires knowledge of the ignition temperatures and combustion rates of different types of wood. Similarly, optimizing wood-burning stoves depends on maximizing the complete combustion of wood to reduce emissions and increase efficiency.
Furthermore, burning wood is a significant source of carbon emissions, contributing to climate change. Understanding the chemistry of combustion helps researchers develop strategies to mitigate these emissions.
Human Interest Angle: Campfire Cooking
For many, burning wood conjures images of cozy campfires and delicious meals cooked over open flames. But even in this simple act, the chemical and physical changes are at play.
The heat from the burning wood cooks the food, while the smoke imparts a unique flavor. Understanding the principles of combustion allows for better control of the fire, ensuring perfectly cooked meals and a safe and enjoyable experience.
Important: Always follow fire safety guidelines when building a campfire and never leave a fire unattended.
Conclusion
In conclusion, the burning of wood is a complex process that involves both chemical and physical changes. While the formation of new substances like carbon dioxide and water definitively marks it as a chemical change, physical transformations such as evaporation and structural breakdown also play a crucial role.
A thorough understanding of these changes is essential for a wide range of applications, from environmental conservation to everyday practices like cooking. By grasping the science behind the flames, we can better manage and mitigate the impacts of this fundamental process.



/burninglogs-58dd324c5f9b584683b72ea0.jpg)








/184391933-56a34a305f9b58b7d0d14e45.jpg)