Charcot Marie Tooth Hereditary Neuropathy Market

Imagine a world where every step requires conscious effort, where simple tasks like buttoning a shirt or walking across a room demand intense concentration. For millions, this isn't a far-off dystopia, but the everyday reality of living with Charcot-Marie-Tooth disease (CMT), a group of inherited disorders that chip away at the nerves controlling muscles.
The Charcot-Marie-Tooth Hereditary Neuropathy Market is undergoing significant evolution, fueled by increased awareness, advancements in genetic testing, and the growing urgency for effective treatments. While currently lacking a cure, the landscape is shifting with burgeoning research and development activities, offering hope for improved management and potentially disease-modifying therapies.
Understanding CMT: A Genetic Puzzle
CMT, named after the three physicians who first described it – Jean-Martin Charcot, Pierre Marie, and Howard Henry Tooth – is one of the most common inherited neurological disorders. It affects approximately 1 in 2,500 individuals worldwide, impacting motor and sensory nerves.
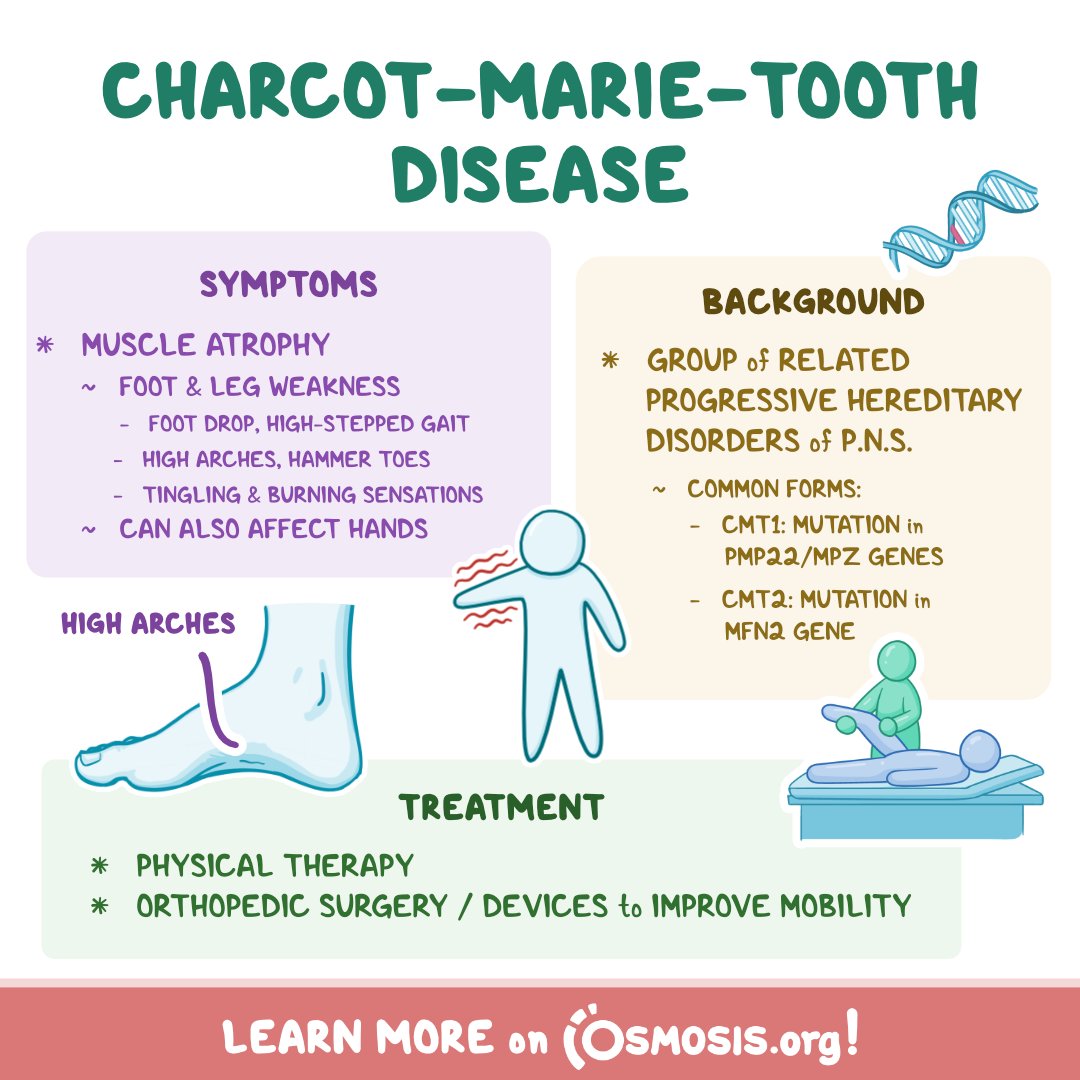
The disease is caused by mutations in genes that affect the structure and function of peripheral nerves, the nerves outside the brain and spinal cord. These mutations disrupt the transmission of signals from the brain to the muscles, leading to muscle weakness and atrophy, particularly in the feet and legs.
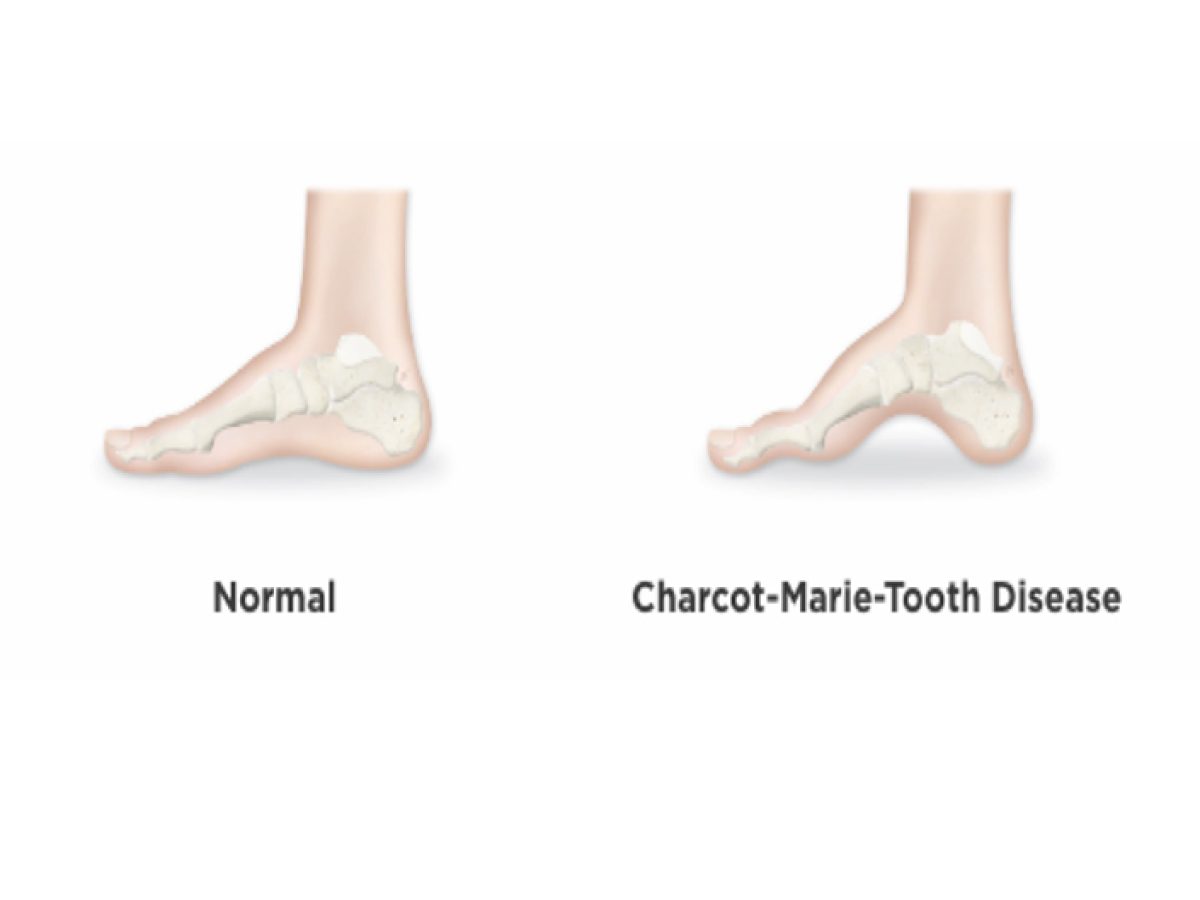
CMT presents with a wide range of symptoms, differing in severity and onset. Common manifestations include foot deformities like high arches or hammertoes, difficulty walking, muscle cramping, and decreased sensation in the extremities. As the disease progresses, it can affect hand function and even breathing in severe cases.
The Market Drivers: Needs and Advances
Several factors are converging to drive the growth of the CMT market. One critical element is the increasing awareness among healthcare professionals and the general public. Improved diagnostic tools, particularly genetic testing, play a significant role in identifying individuals with CMT early in their lives, creating a greater demand for management strategies.
The rising prevalence of CMT, coupled with an aging global population, contributes to the growing patient pool seeking treatment. Furthermore, government initiatives and support from patient advocacy groups stimulate research funding and promote the development of new therapies. Patient advocacy groups like the CMTA (Charcot-Marie-Tooth Association) are instrumental in driving research and providing support to families affected by CMT.
The research and development pipeline for CMT treatments is expanding, offering glimpses of hope. Various therapeutic approaches are under investigation, including gene therapy, small molecule drugs, and cell-based therapies. These approaches aim to either correct the underlying genetic defect or alleviate the symptoms of the disease.
Challenges and Opportunities
Despite the growing momentum, several challenges remain in the CMT market. One of the biggest hurdles is the genetic heterogeneity of the disease. With over 100 different genes implicated in CMT, developing a single, universally effective treatment is a formidable task. Personalized medicine approaches tailored to specific genetic mutations may prove more fruitful.
Another challenge is the lack of well-defined clinical endpoints for measuring treatment efficacy. This makes it difficult to assess the effectiveness of new therapies in clinical trials. Developing sensitive and reliable outcome measures is crucial for accelerating drug development.
Nevertheless, these challenges also present opportunities. Advances in genetic engineering, such as CRISPR-Cas9 technology, hold immense potential for correcting genetic mutations that cause CMT. Novel drug delivery systems, like nanoparticles, could improve the bioavailability and targeted delivery of therapeutic agents to the affected nerves.
The Current Landscape: Management and Emerging Therapies
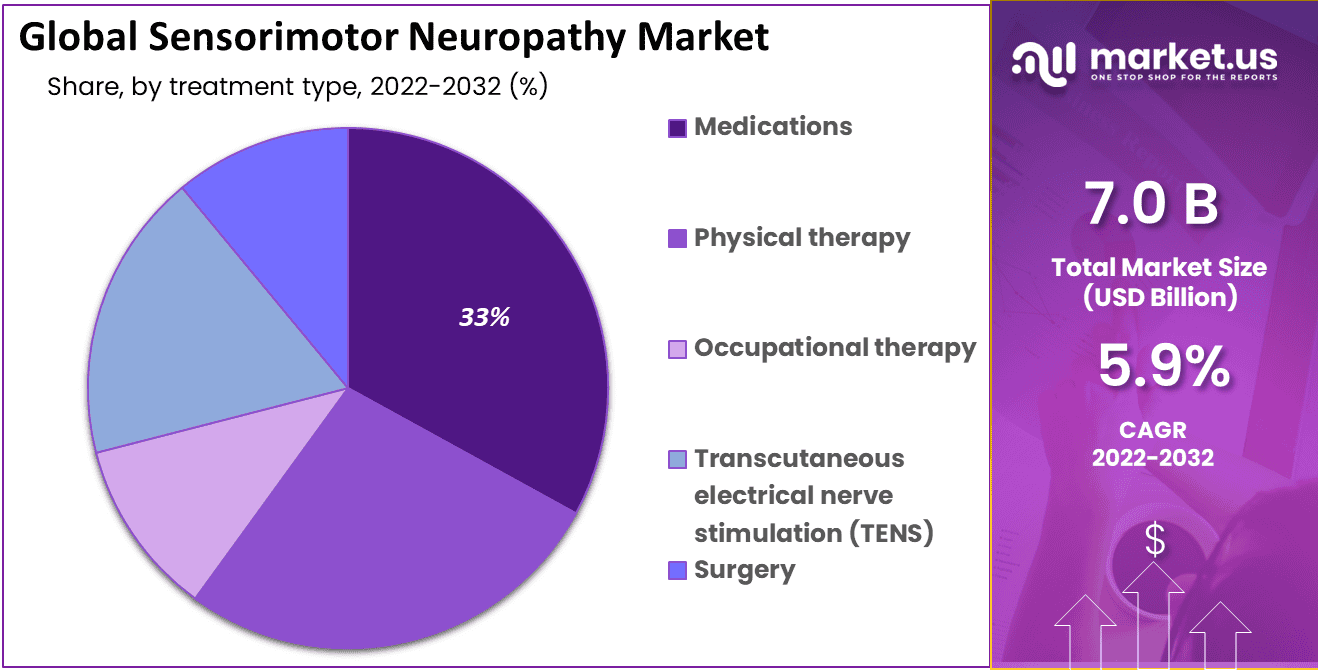
Currently, CMT management focuses on supportive care, aimed at alleviating symptoms and improving quality of life. This includes physical therapy to maintain muscle strength and flexibility, occupational therapy to adapt to daily activities, and orthotics to support the feet and ankles.
Pain management is also a crucial aspect of CMT care. Patients may experience chronic pain due to nerve damage and muscle cramping. Medications, such as analgesics and anticonvulsants, can help alleviate pain and improve sleep quality.
While there is no cure, the development of disease-modifying therapies is rapidly advancing. Several companies and research institutions are actively pursuing novel therapeutic approaches. Gene therapy, which aims to replace or repair the defective genes, is showing promise in preclinical studies.
Small molecule drugs that target specific pathways involved in nerve degeneration are also under development. These drugs aim to protect the nerves from damage and promote their regeneration. Cell-based therapies, such as stem cell transplantation, are being investigated as a potential way to replace damaged nerve cells.
Market Segmentation: A Complex Picture
The CMT market can be segmented based on several factors. One is by the type of CMT, such as CMT1A, CMT1X, and CMT2. CMT1A, caused by a duplication of the PMP22 gene, is the most common type of CMT.
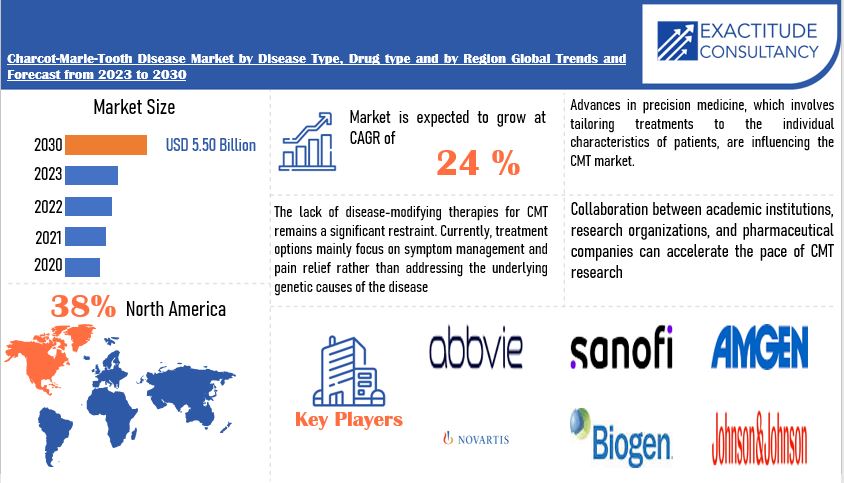
The market can also be segmented by the type of therapy, such as gene therapy, small molecule drugs, and cell-based therapies. Another segmentation is by geography, with North America, Europe, and Asia Pacific being the major regions.
North America currently dominates the CMT market, due to its advanced healthcare infrastructure and high awareness of the disease. However, Asia Pacific is expected to witness the fastest growth rate, driven by increasing healthcare spending and improving diagnostic capabilities.
Looking Ahead: Hope and Innovation
The future of the CMT market is filled with both challenges and opportunities. As research progresses and new therapies emerge, there is growing hope for improved management and potentially disease-modifying treatments. Advances in genetic testing and personalized medicine approaches will enable more targeted and effective therapies.
Increased collaboration between researchers, clinicians, and pharmaceutical companies is essential to accelerate drug development. Patient advocacy groups will continue to play a vital role in raising awareness, providing support, and advocating for research funding. The unwavering spirit of those affected by CMT, combined with scientific innovation, paves the way for a brighter future.
The journey for those living with CMT is undoubtedly challenging, but it's not without hope. As the scientific community delves deeper into the complexities of this genetic puzzle, the promise of effective treatments and a better quality of life for millions with CMT becomes increasingly tangible. It's a testament to human ingenuity and the power of collective effort to alleviate suffering and enhance the human experience.