Content Of Human Factors Information In Medical Device Marketing Submissions

Imagine a bustling hospital room, the air thick with the quiet hum of machines. A nurse, weary from a long shift, reaches for a new infusion pump. But the on-screen instructions are confusing, the buttons too small, the interface unintuitive. A critical moment stretches into an agonizing delay, a delay that could have serious consequences.
This scenario, unfortunately, isn't just a hypothetical. It underscores a critical issue in medical device design: the inclusion of robust human factors information in marketing submissions. This article delves into the importance of this data, exploring how it ensures medical devices are safe, effective, and user-friendly, ultimately protecting patients and healthcare professionals alike.
The Crucial Role of Human Factors
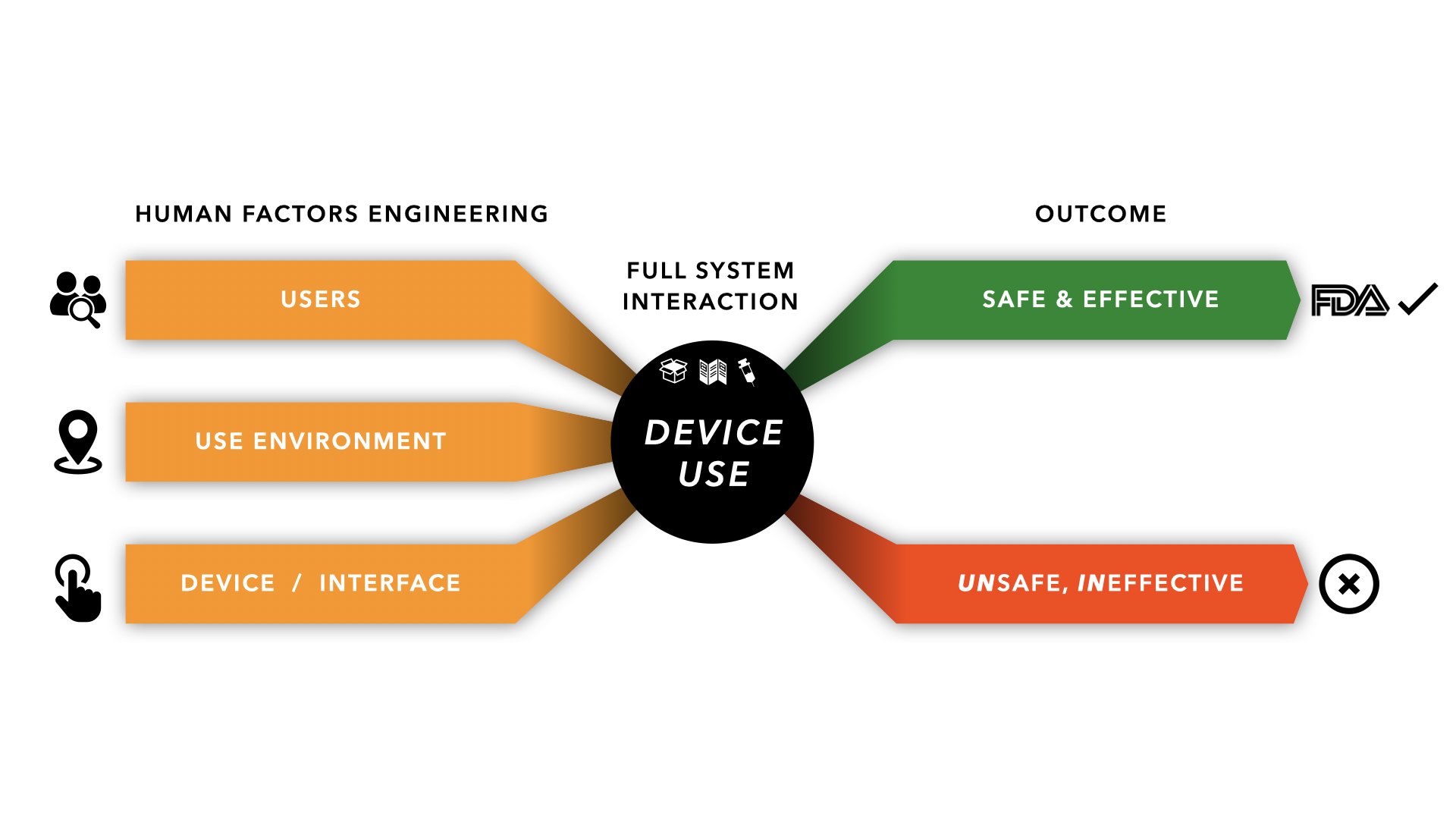
Human factors, also known as usability engineering, focuses on the interaction between humans and products. In the context of medical devices, it's about understanding how healthcare professionals and patients will use the device, and designing it to minimize errors and maximize efficiency.
This involves considering a range of factors, from the physical ergonomics of the device to the clarity of the user interface and the cognitive demands placed on the user. Ignoring these factors can lead to device misuse, delayed treatment, and even patient harm. Robust human factors data in marketing submissions helps regulatory bodies like the FDA assess whether a device is safe and effective for its intended users.
The consequences of neglecting human factors can be dire. Consider the infamous case of the Therac-25, a radiation therapy machine that delivered massive overdoses of radiation due to software errors and a poorly designed user interface. This tragedy highlighted the critical need for thorough human factors testing and a user-centered design approach.
What Constitutes Adequate Human Factors Information?
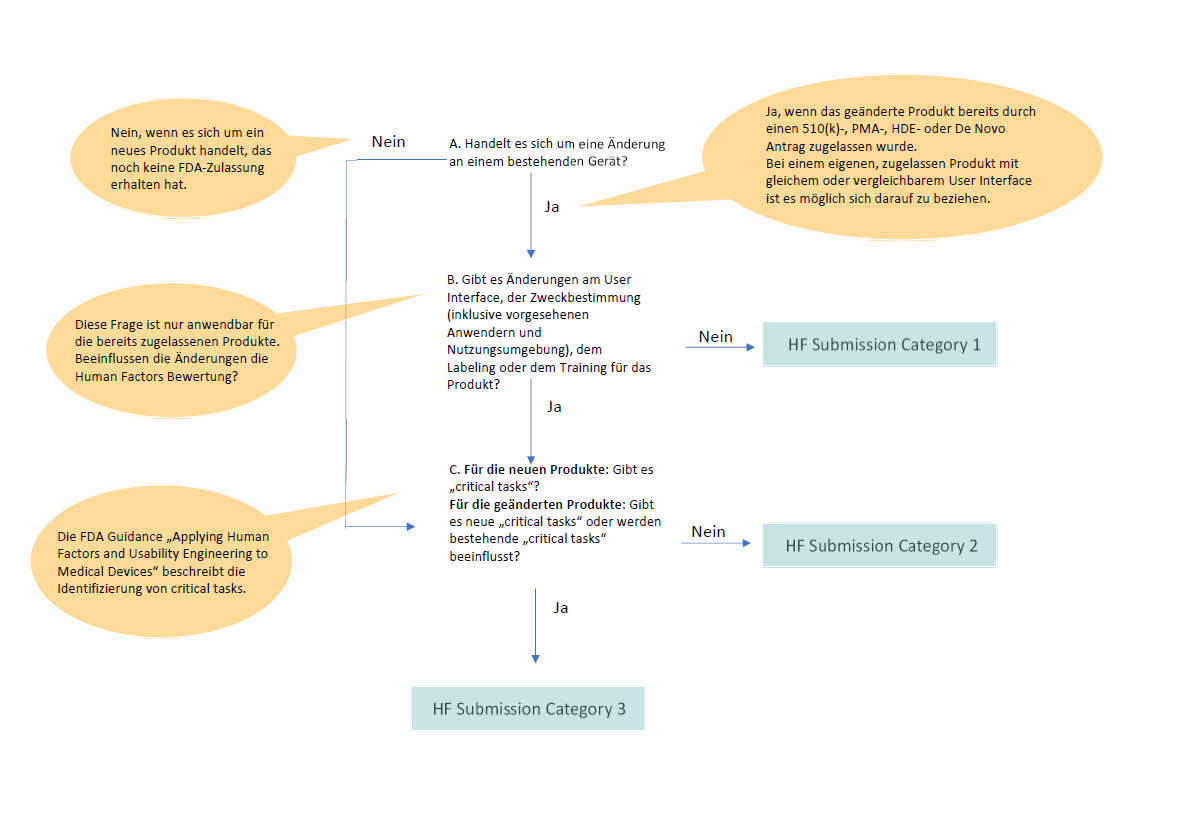
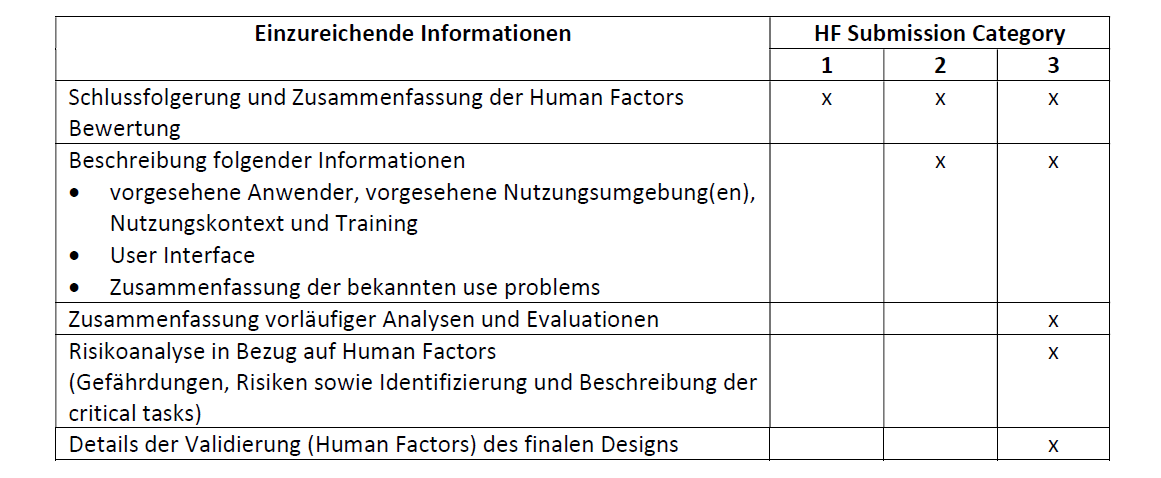
So, what exactly should manufacturers include in their medical device marketing submissions to demonstrate adequate consideration of human factors? The FDA provides detailed guidance on this topic, outlining the types of information that are essential for a thorough review.
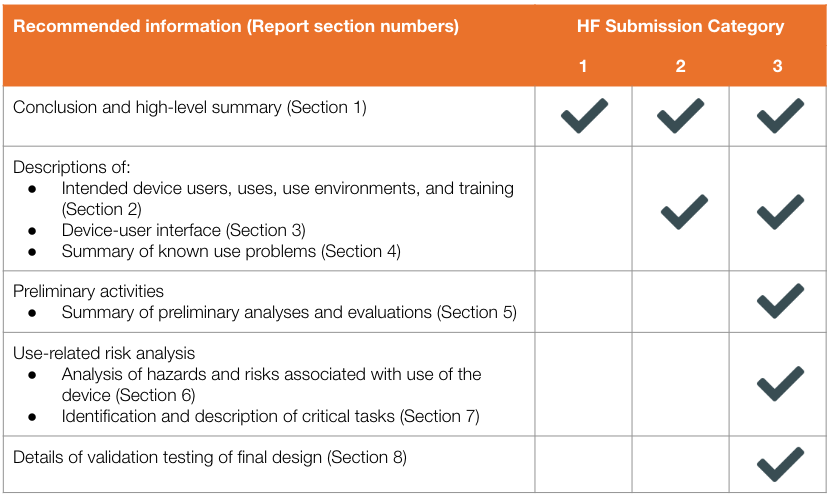
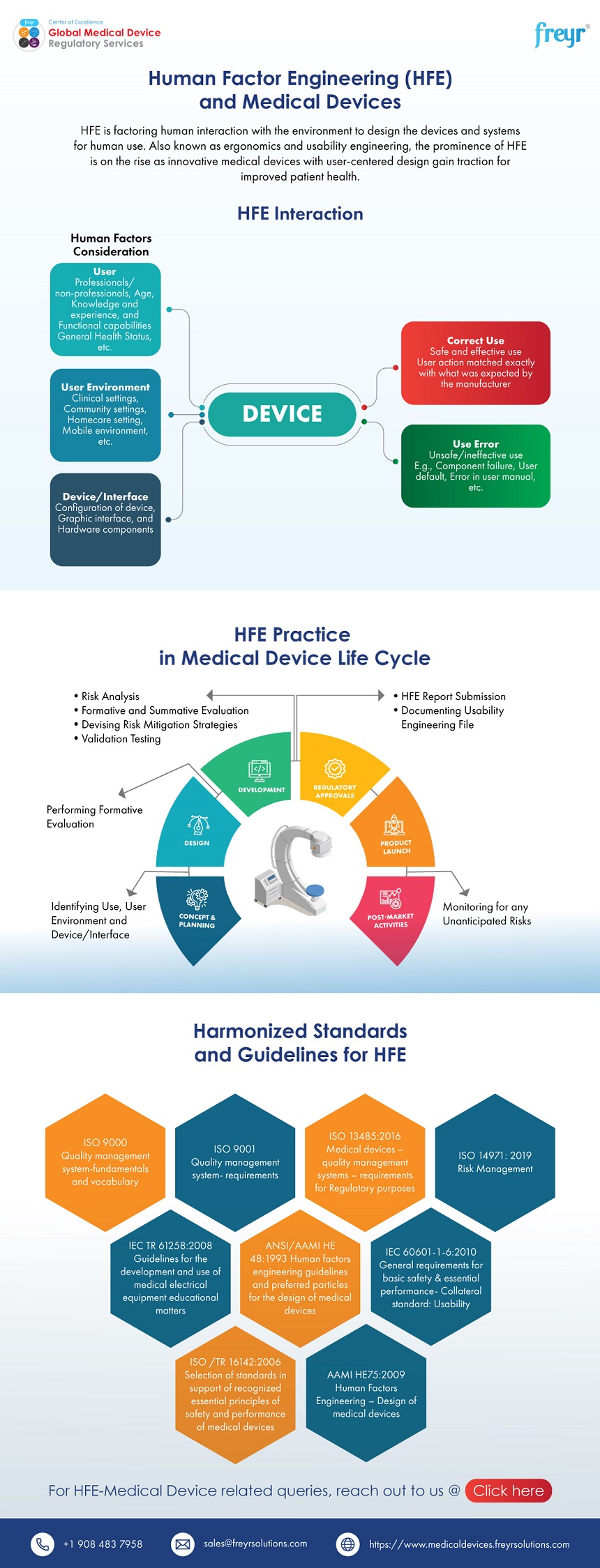
This includes a detailed description of the device's intended users, use environments, and use scenarios. It also requires a comprehensive hazard analysis, identifying potential use-related hazards and the steps taken to mitigate them. Formative and summative usability testing data are also crucial components.
Formative testing involves iterative testing throughout the design process, allowing manufacturers to identify and address usability issues early on. Summative testing, on the other hand, is conducted on the final device to evaluate its overall usability and safety.
The FDA also expects manufacturers to provide evidence of a user-centered design process. This means involving end-users in the design process, incorporating their feedback into the device's design, and documenting all design changes and rationale.
FDA Guidance and Expectations
The FDA's Center for Devices and Radiological Health (CDRH) has published extensive guidance documents on human factors engineering for medical devices. These documents outline the agency's expectations for human factors testing and documentation. The agency's commitment to human factors is evident in its increasing scrutiny of marketing submissions and its willingness to request additional information when necessary.
According to the FDA's website, "Human factors engineering (HFE) is the application of knowledge about human capabilities and limitations to the design of interactive systems, such as medical devices and associated packaging." This definition underscores the agency's belief that medical device design must be grounded in a thorough understanding of human behavior and cognition.
The FDA emphasizes the importance of a risk-based approach to human factors engineering. This means that manufacturers should focus their efforts on identifying and mitigating the most critical use-related hazards. Devices that pose a higher risk to patients require more rigorous human factors testing.
The Benefits of Prioritizing Human Factors
Prioritizing human factors in medical device design isn't just about compliance with regulatory requirements. It's also about improving patient safety, enhancing healthcare professional efficiency, and reducing the risk of device-related errors.
Well-designed medical devices are easier to use, require less training, and are less prone to errors. This can lead to improved patient outcomes, reduced healthcare costs, and a more positive user experience. It also fosters a culture of safety within healthcare organizations, where errors are minimized and patients are protected.
Moreover, investing in human factors can provide a competitive advantage. Devices that are known for their usability and safety are more likely to be adopted by healthcare professionals and patients. This can lead to increased sales and market share.
Challenges and Future Directions
Despite the growing recognition of the importance of human factors, there are still challenges to overcome. One challenge is the lack of standardized methods for assessing usability. While the FDA provides guidance, manufacturers often rely on their own internal processes and expertise.
Another challenge is the cost of human factors testing. Thorough testing can be expensive, particularly for small companies with limited resources. However, the costs of neglecting human factors can be far greater, including product recalls, lawsuits, and damage to reputation.
Looking ahead, there is a growing trend towards incorporating more advanced technologies into medical device design, such as artificial intelligence and machine learning. These technologies have the potential to improve the usability and safety of medical devices, but they also introduce new human factors challenges.
For example, AI-powered diagnostic tools may require users to interpret complex algorithms and visualizations. It's crucial to ensure that these tools are designed in a way that is intuitive and easy to understand, minimizing the risk of misinterpretation and errors.
"The future of medical device design lies in a user-centered approach that integrates human factors engineering from the very beginning. By prioritizing usability and safety, we can create devices that are truly beneficial to patients and healthcare professionals alike," notes Dr. Emily Carter, a leading expert in human factors.
Increased collaboration between engineers, clinicians, and human factors specialists will be essential to address these challenges and ensure that medical devices are designed with the end-user in mind.
A Final Thought
The inclusion of comprehensive human factors information in medical device marketing submissions is more than just a regulatory requirement; it's a moral imperative. By prioritizing usability and safety, we can create a healthcare system where medical devices are tools that empower healthcare professionals and improve patient outcomes, rather than sources of frustration and error. Let us strive to create a future where every medical device is designed with the user in mind, ensuring that technology serves humanity, not the other way around. A safe and effective medical device is not just a product; it's a promise kept.







![Content Of Human Factors Information In Medical Device Marketing Submissions Medical Device Marketing Is Trending Right Now [INFOGRAPHIC]](https://tycoonstorymedia.b-cdn.net/wp-content/uploads/2022/04/Why-Medical-Device-Marketing-Is-Trending-Right-Now-Tycoonstory.jpg)