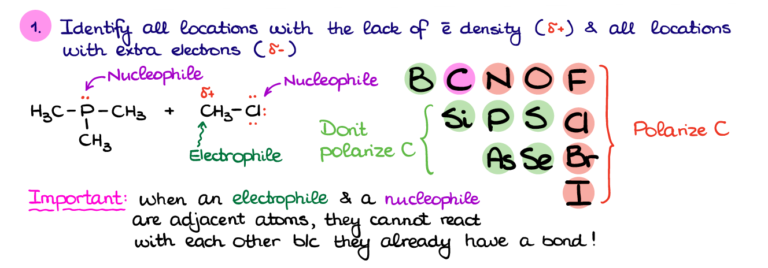Define Nucleophile And Electrophile With Example
Imagine a bustling dance floor, where molecules are the dancers. Some are eager to give away partners, while others are desperate to find one. This chemical courtship plays out on a microscopic level, governed by the attractions between nucleophiles and electrophiles. These terms might sound intimidating, but understanding them unlocks a fundamental key to understanding how chemical reactions occur.
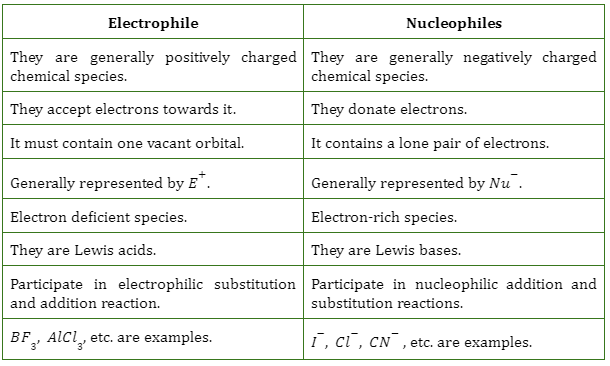
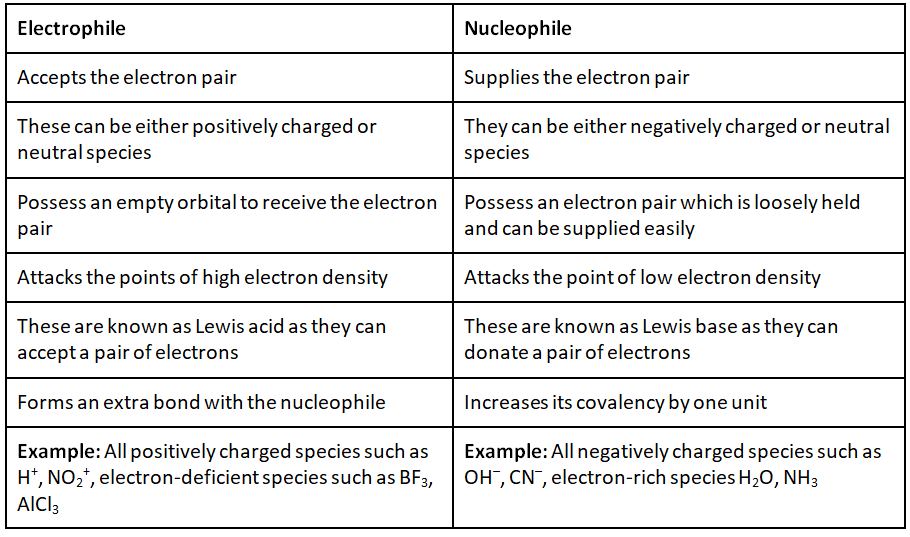
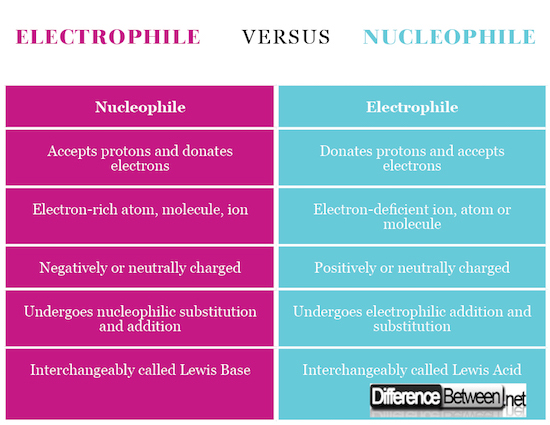
At its core, the concepts of nucleophiles and electrophiles explain the 'who' and 'why' of chemical reactions. A nucleophile is an electron-rich species seeking a positive charge, while an electrophile is electron-deficient and craves electrons. This article aims to demystify these terms, providing clear definitions, illustrative examples, and an understanding of their significance in the world of chemistry.
Nucleophiles: The Electron Lovers
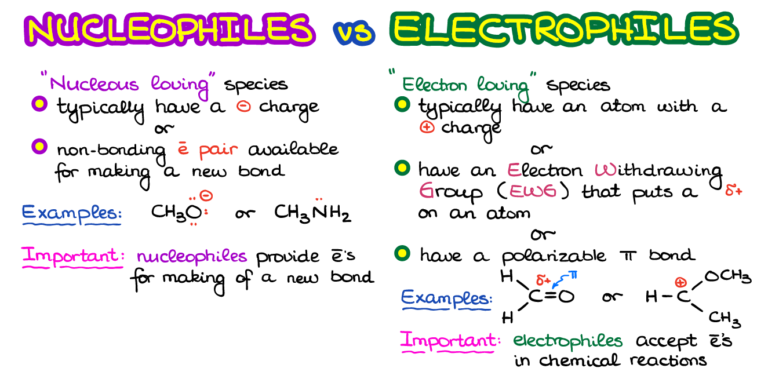
The word "nucleophile" comes from the Greek words "nucleus" (meaning "kernel" or "center") and "phile" (meaning "loving"). A nucleophile, therefore, is a species that loves the nucleus – specifically, a nucleus bearing a positive charge.
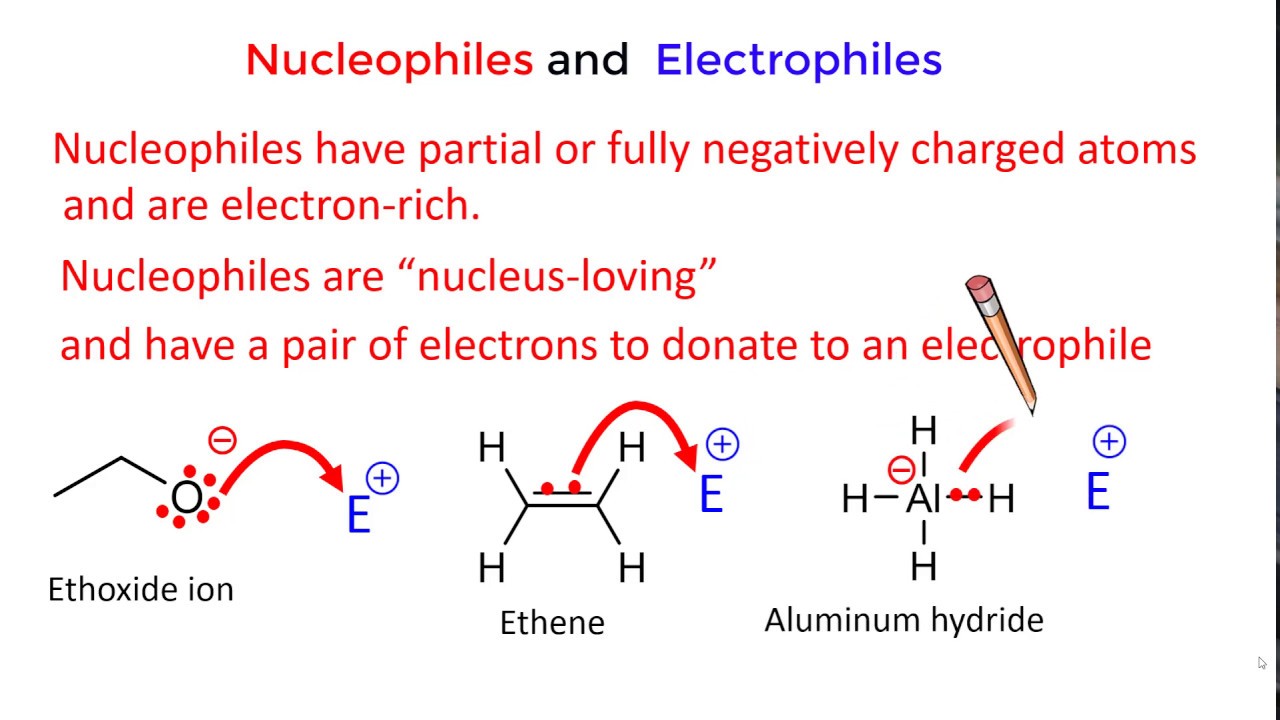
In chemical terms, a nucleophile is an electron-rich atom or molecule that is attracted to a positive charge and participates in a chemical reaction by donating electrons to it. Nucleophiles are typically anions (negatively charged ions) or molecules with lone pairs of electrons.
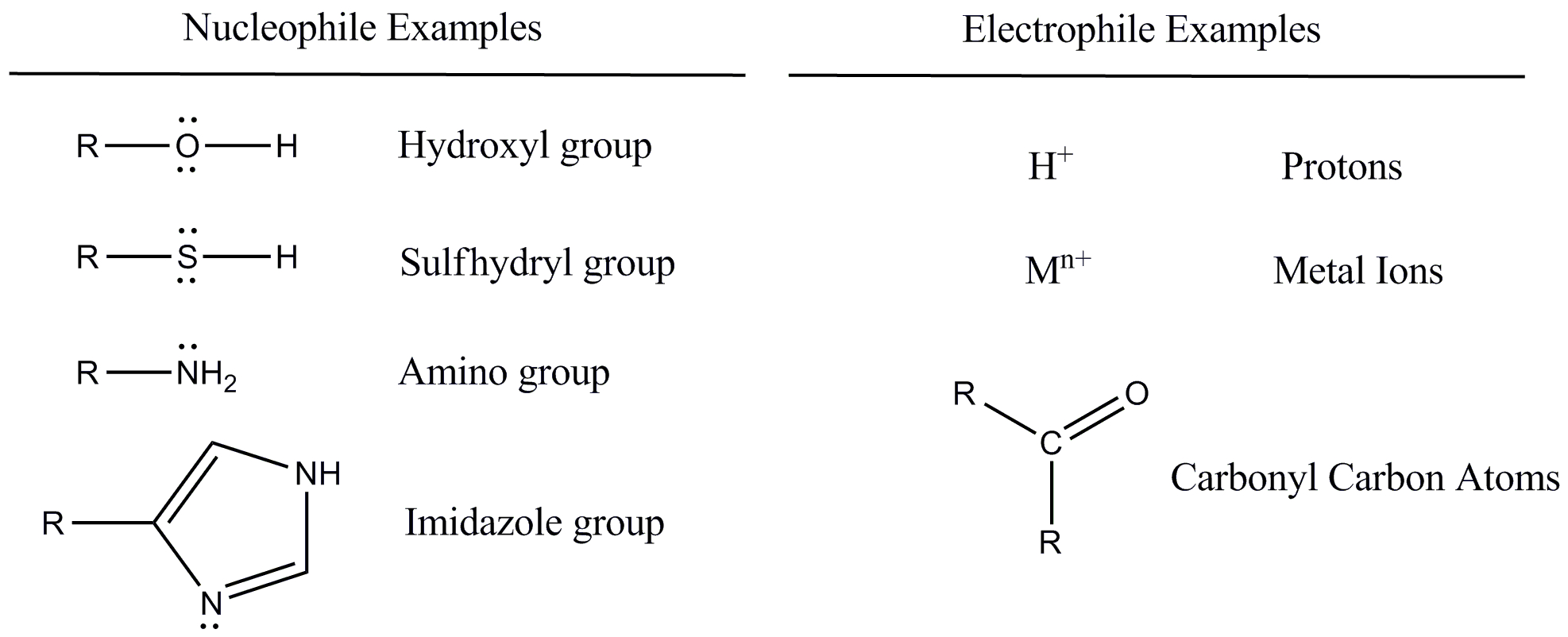
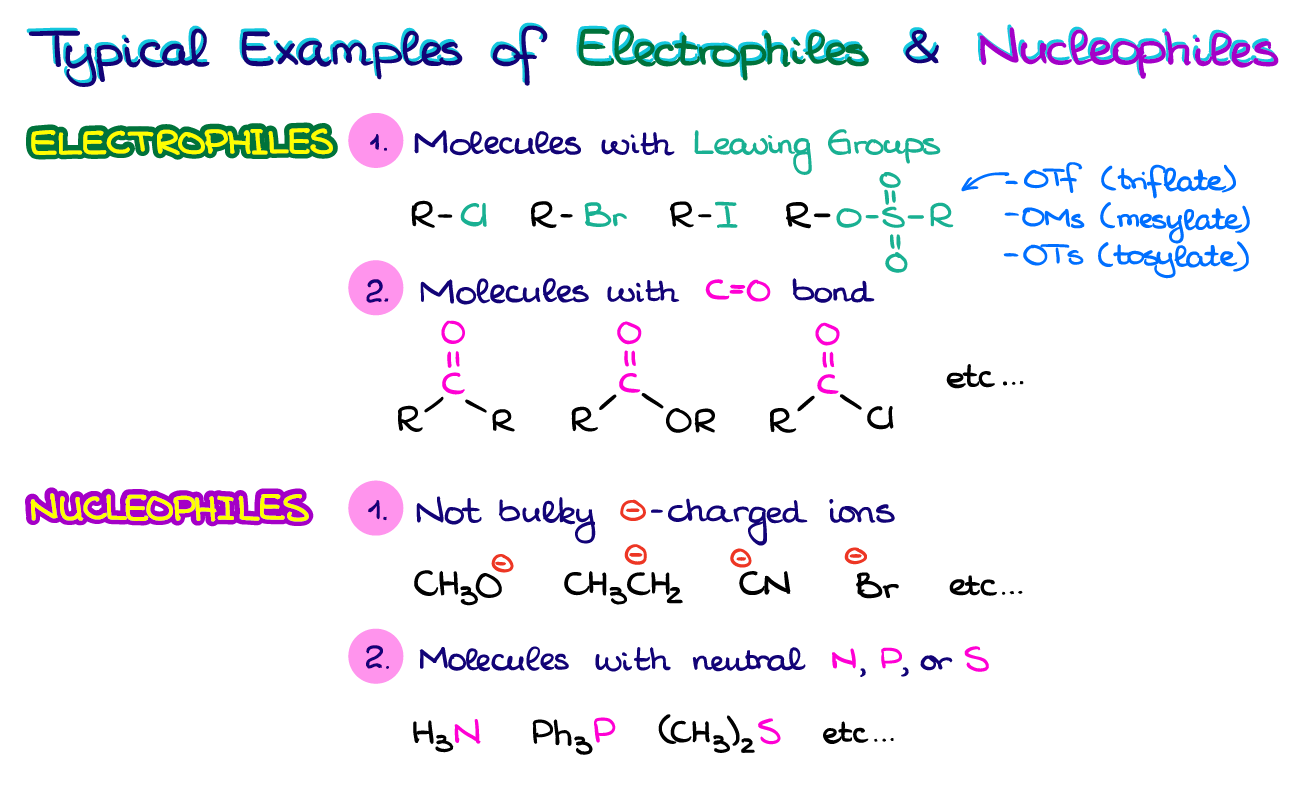
Examples of common nucleophiles include hydroxide ions (OH⁻), ammonia (NH₃), and cyanide ions (CN⁻). These species possess an abundance of electrons that they are willing to share.
Examples in Action
Consider the reaction of hydroxide ions (OH⁻) with methyl bromide (CH₃Br). The hydroxide ion, a strong nucleophile, attacks the electron-deficient carbon atom in methyl bromide.
This attack leads to the displacement of the bromide ion (Br⁻), resulting in the formation of methanol (CH₃OH). This is a classic example of a nucleophilic substitution reaction, where the nucleophile (OH⁻) replaces another group (Br⁻).
Electrophiles: The Electron Seekers
Electrophiles, on the other hand, are electron-deficient species that are attracted to negative charges. Their name also comes from Greek words "electro" (related to electrons) and "phile" (loving).
Essentially, an electrophile is "electron-loving" and seeks out electrons to achieve a more stable electronic configuration. They are typically cations (positively charged ions) or molecules with incomplete octets of electrons.
Examples of common electrophiles include carbocations (positively charged carbon ions), protons (H⁺), and carbonyl compounds (containing a C=O group). These species have a strong desire for electrons to fill their electronic shells.
Examples in Action
A simple example involves the reaction of a proton (H⁺), a strong electrophile, with ammonia (NH₃). Ammonia has a lone pair of electrons on the nitrogen atom.
The proton, craving electrons, is attracted to this lone pair and forms a coordinate covalent bond with the nitrogen, creating the ammonium ion (NH₄⁺). This demonstrates how electrophiles accept electrons from nucleophiles.
The Significance of Nucleophiles and Electrophiles
The concepts of nucleophiles and electrophiles are fundamental to understanding a vast range of organic reactions. They provide a framework for predicting the outcome of reactions and designing new synthetic strategies.
Pharmaceuticals, polymers, and countless other materials are synthesized using reactions that involve the interplay of nucleophiles and electrophiles. Understanding these interactions allows chemists to control the formation of new chemical bonds and create molecules with specific properties.
In biochemistry, enzyme-catalyzed reactions often involve nucleophilic and electrophilic attacks. Enzymes act as catalysts by providing specific environments that facilitate these interactions, enabling biological processes to occur at remarkable speeds.
Conclusion
The dance between nucleophiles and electrophiles is a fundamental aspect of chemistry. By understanding their roles and interactions, we gain a deeper insight into the world of molecules and reactions.
These seemingly simple concepts unlock a powerful framework for understanding the complexity of chemical transformations, paving the way for new discoveries and innovations in fields ranging from medicine to materials science. As we continue to explore the molecular world, the principles of nucleophiles and electrophiles will undoubtedly remain central to our understanding.