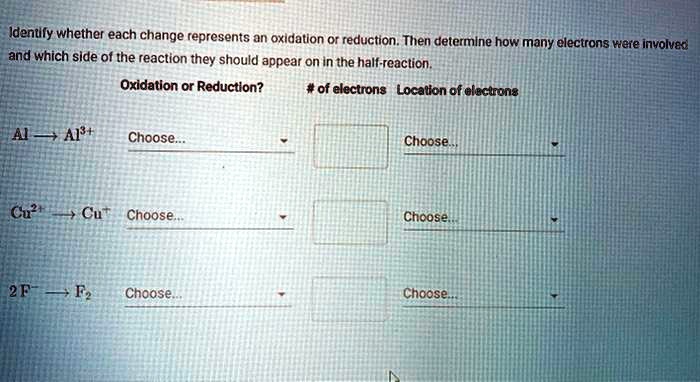
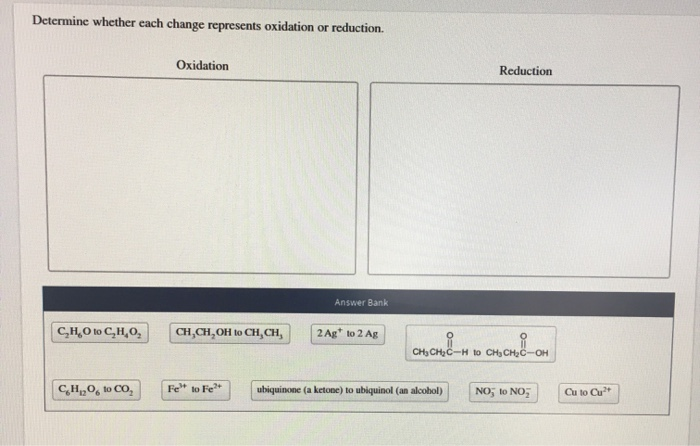
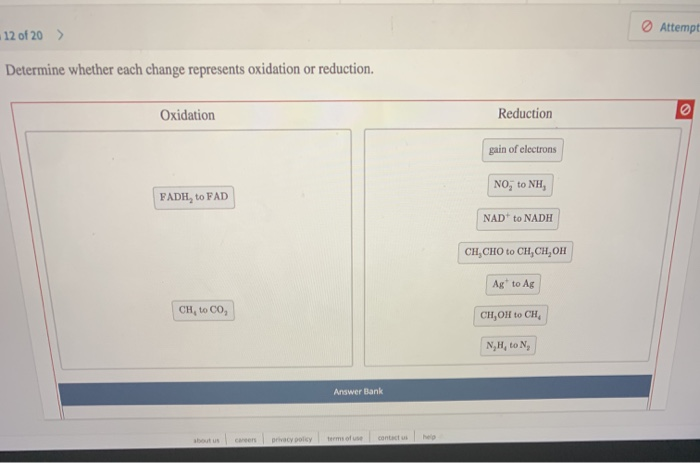
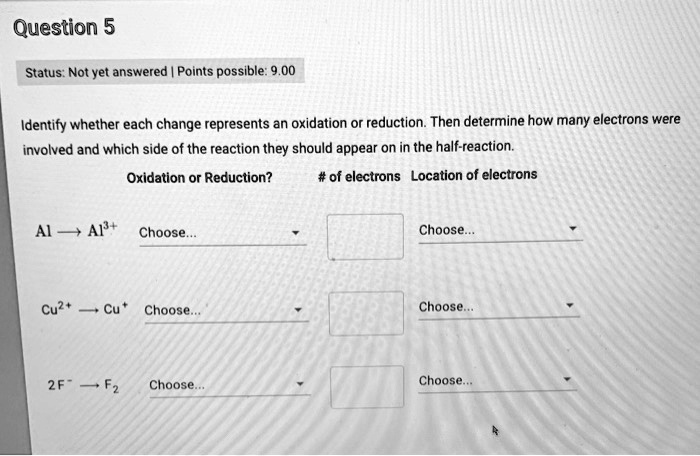
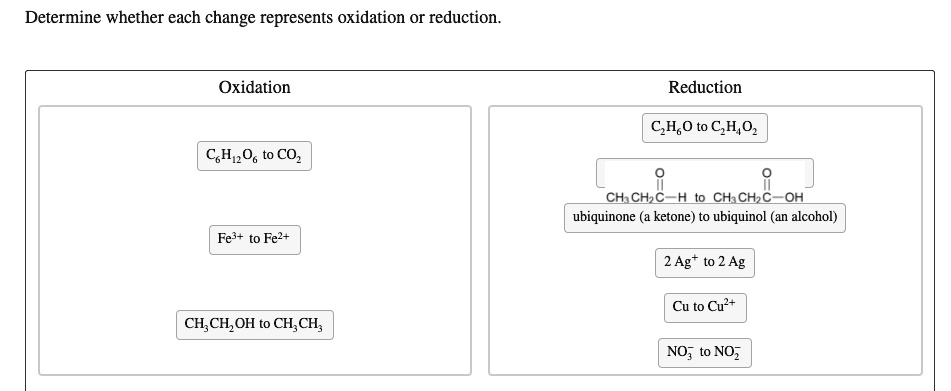
Determine Whether Each Change Represents Oxidation Or Reduction.

Imagine a world where rust never formed on metal, where batteries never ran down, and where the vibrant green of leaves never faded. These everyday phenomena, seemingly disparate, are all governed by the same fundamental principle: redox reactions.
This article aims to provide a clear and accessible guide to understanding oxidation and reduction, empowering you to identify these processes in various contexts, from the mundane to the complex.
Understanding the Basics of Redox Reactions
At its core, a redox reaction, short for reduction-oxidation reaction, involves the transfer of electrons between chemical species. Oxidation, in simple terms, is the loss of electrons, while reduction is the gain of electrons.
To easily remember these concepts, think of the mnemonic "OIL RIG": Oxidation Is Loss, Reduction Is Gain (of electrons).
Oxidation: The Loss of Electrons
When a substance is oxidized, it loses electrons, resulting in an increase in its oxidation number. This process often involves the addition of oxygen or the removal of hydrogen.
For example, when iron rusts, it's being oxidized. The iron atoms lose electrons to oxygen atoms in the air, forming iron oxide (rust). This is a classic example of oxidation in action.
Reduction: The Gain of Electrons
Conversely, when a substance is reduced, it gains electrons, resulting in a decrease in its oxidation number. Reduction often involves the removal of oxygen or the addition of hydrogen.
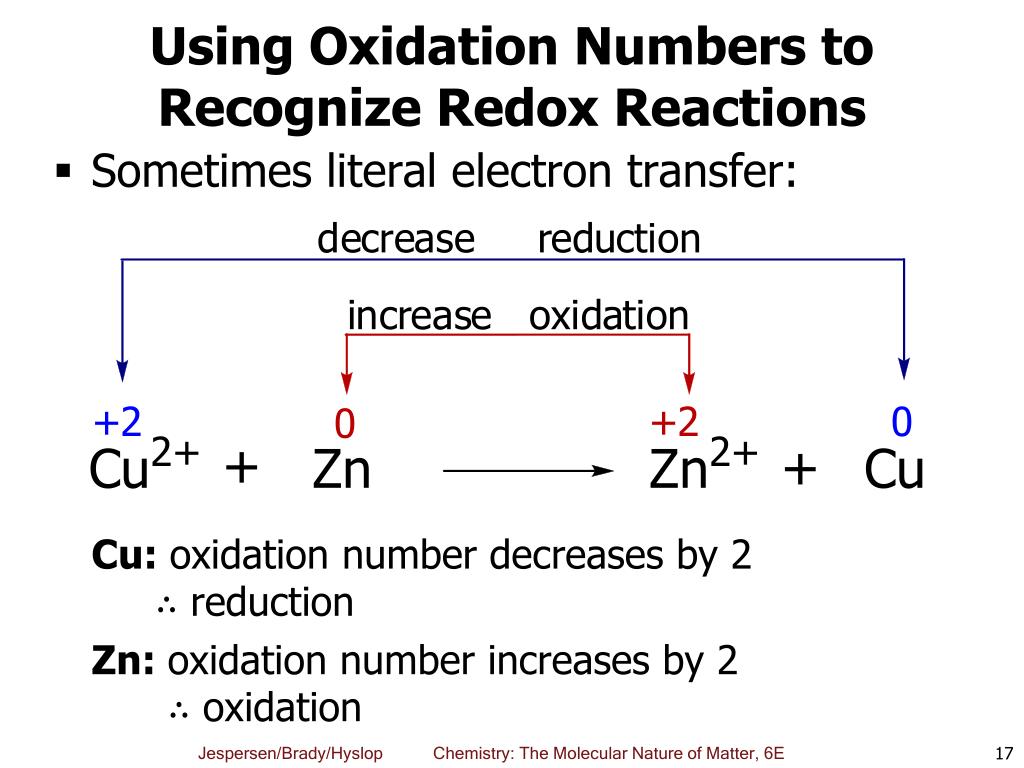
Consider the process of copper ions (Cu2+) in a solution reacting with zinc metal (Zn). The copper ions gain electrons from the zinc, becoming solid copper metal (Cu), while the zinc atoms lose electrons and become zinc ions (Zn2+). The copper ions are reduced, and the zinc metal is oxidized.
Identifying Oxidation and Reduction in Chemical Equations
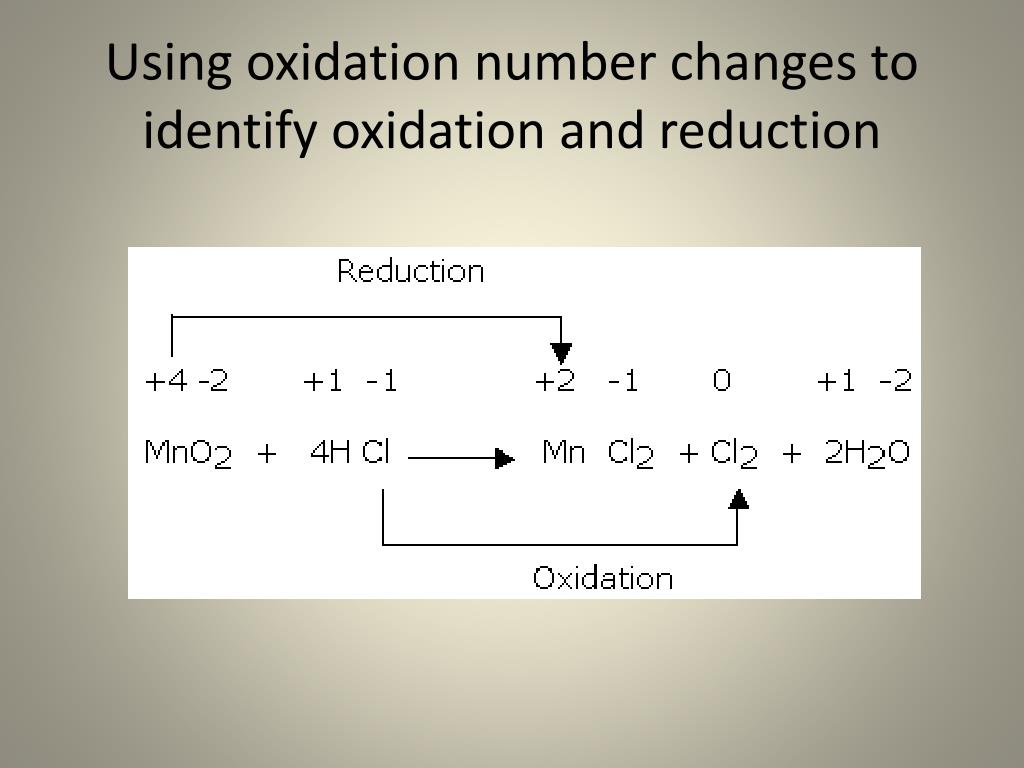
To determine whether a change represents oxidation or reduction, we need to examine the oxidation numbers of the atoms involved.
Oxidation number, also known as oxidation state, is a number assigned to an element in a chemical compound that represents the number of electrons it has gained or lost compared to its neutral state. A change in oxidation number indicates whether oxidation or reduction has occurred.
Rules for Assigning Oxidation Numbers
Several rules govern the assignment of oxidation numbers:
1. The oxidation number of an atom in its elemental form is always 0 (e.g., Fe, O2, N2).
2. The oxidation number of a monatomic ion is equal to its charge (e.g., Na+ is +1, Cl- is -1).
3. The sum of the oxidation numbers of all atoms in a neutral molecule is 0.
4. The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
5. In compounds, alkali metals (Group 1) have an oxidation number of +1, and alkaline earth metals (Group 2) have an oxidation number of +2.
6. Fluorine always has an oxidation number of -1 in compounds.
7. Oxygen usually has an oxidation number of -2 in compounds, except in peroxides (e.g., H2O2) where it is -1 and when combined with fluorine where it is positive.
8. Hydrogen usually has an oxidation number of +1 in compounds, except when bonded to metals, where it is -1 (e.g., NaH).
Applying the Rules: Examples
Let's analyze a few reactions:
Example 1: 2Mg(s) + O2(g) → 2MgO(s)
Magnesium (Mg) goes from an oxidation number of 0 to +2 (since O is -2 in MgO and the compound is neutral, Mg must be +2). Oxygen (O) goes from an oxidation number of 0 to -2. Magnesium is oxidized (loses electrons), and oxygen is reduced (gains electrons).
Example 2: Cu2+(aq) + Zn(s) → Cu(s) + Zn2+(aq)
Copper (Cu) goes from an oxidation number of +2 to 0. Zinc (Zn) goes from an oxidation number of 0 to +2. Copper is reduced (gains electrons), and zinc is oxidized (loses electrons).
Example 3: CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
In CH4, carbon has an oxidation number of -4. In CO2, carbon has an oxidation number of +4. Therefore, carbon is oxidized. Oxygen goes from 0 in O2 to -2 in both CO2 and H2O, so oxygen is reduced.
The Significance of Redox Reactions
Redox reactions are ubiquitous and fundamental to many biological and industrial processes. From the energy production in our cells to the synthesis of vital chemicals, they play a crucial role in sustaining life and driving technological advancements.
Photosynthesis, the process by which plants convert sunlight into energy, is a complex series of redox reactions. Respiration, the process by which animals obtain energy from food, also involves redox reactions.
Industrially, redox reactions are used in the production of metals, the manufacturing of fertilizers, and the purification of water. Batteries rely on controlled redox reactions to generate electricity. Even the bleaching of fabrics involves redox chemistry.
Real-World Applications and Examples
Consider these applications:
Corrosion: The rusting of iron, as mentioned earlier, is a detrimental redox reaction. Protecting metals from corrosion often involves preventing oxidation, such as by coating them with paint or other protective layers.
Batteries: Batteries utilize redox reactions to convert chemical energy into electrical energy. Different battery types use different redox couples, each offering varying voltage and energy density.
Combustion: Burning fuels like wood or natural gas involves rapid redox reactions between the fuel and oxygen, releasing heat and light.
Bleaching: Bleach, often containing sodium hypochlorite (NaClO), uses redox reactions to break down colored compounds, removing stains from fabrics and other materials.
Balancing Redox Reactions
Balancing redox reactions is essential to ensure that the number of atoms and charges are equal on both sides of the equation. There are two common methods for balancing redox reactions: the half-reaction method and the oxidation number method.
The half-reaction method involves separating the overall reaction into two half-reactions: an oxidation half-reaction and a reduction half-reaction. Each half-reaction is balanced separately, and then the two half-reactions are combined to give the balanced overall reaction.
The oxidation number method involves assigning oxidation numbers to all atoms in the reaction and then using the changes in oxidation numbers to balance the equation. This method is particularly useful for reactions that are difficult to balance using the half-reaction method.
Conclusion: Embracing the Dance of Electrons
Understanding oxidation and reduction opens a window into the intricate world of chemical reactions that shape our environment and drive countless technological innovations. Recognizing the transfer of electrons empowers us to comprehend the mechanisms behind processes that sustain life and advance our society.
By mastering the principles of oxidation numbers and redox reactions, we gain a deeper appreciation for the fundamental forces that govern the universe around us. It's a dance of electrons, constantly shifting and transforming matter, a silent symphony that underpins the very fabric of reality.