Dissolving Salt In Water Is A Physical Change

In kitchens and laboratories worldwide, a seemingly simple act occurs countless times daily: salt dissolving in water. But beneath this commonplace phenomenon lies a fundamental question that has engaged scientists and students alike for generations: Is dissolving salt in water a physical change, or something more complex?
While the answer appears straightforward – it's generally categorized as a physical change – the nuances involved reveal the intricate nature of matter and its interactions. Understanding these processes is crucial not only for grasping basic scientific principles but also for applications ranging from food chemistry to industrial processes.
The Nut Graf: Defining Physical Change
This article will delve into the scientific consensus surrounding the dissolution of salt in water, presenting arguments and evidence supporting its classification as a physical change. We will explore the molecular mechanisms at play, considering the role of water molecules and the preservation of the salt's chemical identity. Furthermore, we will touch upon related concepts like chemical changes and phase transitions, highlighting the distinctions and similarities that often lead to confusion.
Experts from various fields, including chemistry and physics, will weigh in on this fundamental topic, providing insights into its broader significance.
Delving into Dissolution: A Physical Perspective
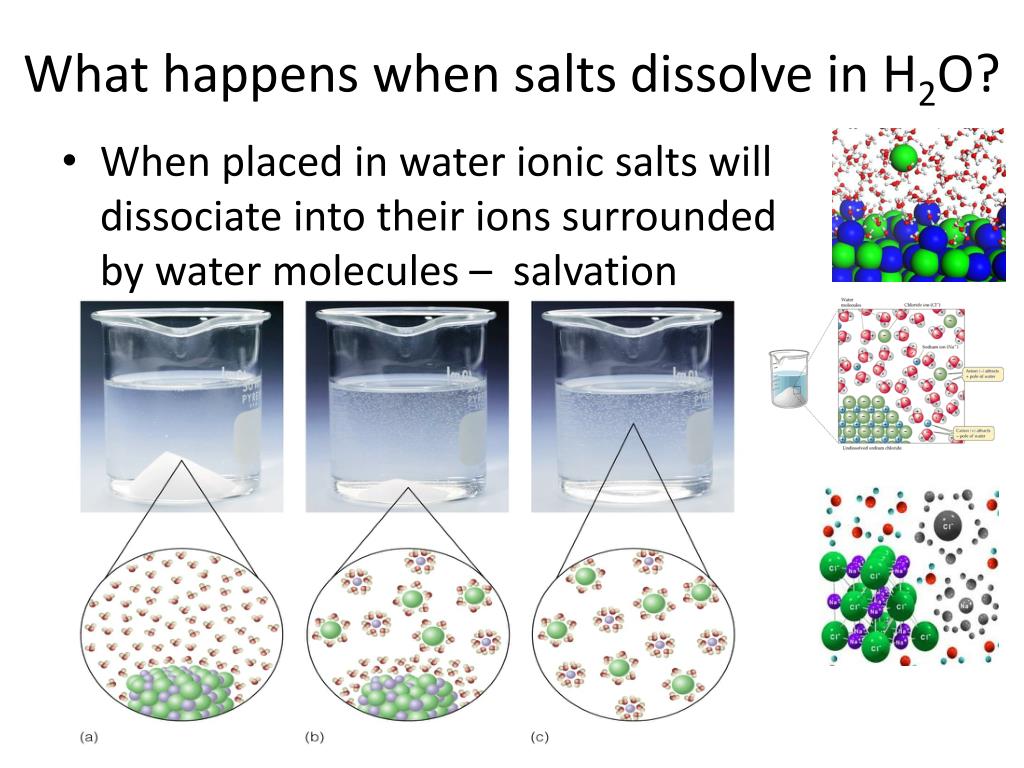
The key argument for classifying salt dissolving in water as a physical change hinges on the fact that the chemical composition of the salt, sodium chloride (NaCl), remains unchanged.
When salt crystals are added to water, the polar water molecules surround the sodium and chloride ions, disrupting the ionic bonds holding the crystal lattice together. This process, known as hydration, separates the ions and disperses them throughout the water.
Crucially, the sodium and chloride ions remain as sodium and chloride ions; they do not transform into new substances. This is a hallmark of a physical change, where the substance's form or appearance is altered, but its fundamental chemical nature remains the same.
Contrasting Physical and Chemical Changes
Understanding the difference between physical and chemical changes is essential for grasping the nature of dissolution.
A chemical change involves the breaking and forming of chemical bonds, resulting in the creation of new substances with different properties.
For example, burning wood is a chemical change because the wood reacts with oxygen to produce ash, carbon dioxide, and water, all substances different from the original wood.
In contrast, dissolving salt in water does not involve the formation of any new chemical bonds or new substances. The salt simply disperses into the water, existing as individual ions.
The Role of Reversibility: Another Key Indicator
Another characteristic often associated with physical changes is reversibility. While not all physical changes are easily reversed, many are, including the dissolution of salt in water.
By evaporating the water, we can recover the original salt crystals, demonstrating that the dissolution process did not fundamentally alter the salt's chemical identity.
This reversibility contrasts sharply with chemical changes, which typically produce substances that cannot be easily converted back to their original forms.
Expert Opinions: A Consensus View
Dr. Emily Carter, a professor of Chemistry at Princeton University, emphasizes the importance of understanding physical changes for students. "Dissolving salt in water is a classic example that helps students distinguish between physical and chemical changes. It is a relatively easy concept that forms a solid foundation for learning about chemical reactions and other more complex phenomena.”
Dr. David Chandler, a physicist at the University of California, Berkeley, adds, "From a physics perspective, the dissolution of salt is a fascinating example of how electrostatic interactions govern the behavior of matter at the molecular level. The hydration of ions is a process that impacts everything from protein folding to the properties of electrolytes."
Statements from reputable organizations, such as the American Chemical Society (ACS), consistently classify the dissolution of ionic compounds like salt as a physical process. ACS publications highlight its use as a prime example in educational materials.
Potential Misconceptions and Nuances
While dissolving salt in water is generally considered a physical change, some nuances and potential misconceptions can arise. For instance, the term 'solution' can sometimes imply the creation of a new entity.
However, in this context, the solution is simply a homogenous mixture where the salt ions are evenly distributed throughout the water. The individual identities of the water and salt are maintained.
Additionally, the process of dissolving can involve some energy changes (e.g., heat absorption or release), which might suggest a chemical process. However, these energy changes are typically associated with the breaking and formation of intermolecular forces, rather than the breaking of chemical bonds within the salt molecules themselves.
Applications and Implications
Understanding the principles behind dissolving salt in water has numerous practical applications.
In food chemistry, it is crucial for controlling the flavor and texture of various dishes. In industrial processes, it plays a vital role in manufacturing chemicals and pharmaceuticals.
Furthermore, understanding the behavior of ions in solution is essential for studying biological systems, where ions play critical roles in nerve impulse transmission and other physiological processes. Seawater desalination depends on this knowledge too.
Looking Ahead: Further Exploration
While the classification of dissolving salt in water as a physical change is well-established, ongoing research continues to explore the complexities of solvation and ionic interactions. Advanced techniques like molecular dynamics simulations are used to model the behavior of ions in solution at the atomic level.
These studies are providing deeper insights into the factors that influence the solubility of different substances and the properties of solutions.
As our understanding of these processes grows, we can expect to see new applications emerge in fields ranging from materials science to environmental engineering, proving the importance of understanding what seems to be a simple concept.