Distinguish Between Ethanol And Phenol

Confusion and misidentification between chemical compounds can have dire consequences, especially in industries ranging from pharmaceuticals to manufacturing. Two substances often mistakenly conflated are ethanol and phenol, both organic compounds but with drastically different properties and applications. Understanding their distinctions is crucial for safety, accuracy, and informed decision-making across various sectors.
This article delves into a detailed comparison of ethanol and phenol, clarifying their chemical structures, properties, uses, and potential hazards. It aims to provide a comprehensive understanding of these two important chemicals, moving beyond superficial similarities to highlight their fundamental differences.
Chemical Structure and Properties
Ethanol (C2H5OH), also known as ethyl alcohol, is a simple aliphatic alcohol. Its structure consists of a two-carbon chain with a hydroxyl (-OH) group attached. This structure gives ethanol its characteristic properties: it is a colorless, volatile, and flammable liquid with a mild, pleasant odor.
Phenol (C6H5OH), on the other hand, is an aromatic organic compound. Its structure features a hydroxyl group directly bonded to a benzene ring. This seemingly minor difference leads to significantly different chemical behavior, as the presence of the aromatic ring affects the reactivity and acidity of the -OH group.
Ethanol is a neutral compound, whereas phenol exhibits weak acidic properties. This acidity arises from the stabilization of the phenoxide ion (C6H5O-) by the benzene ring after the proton (H+) is released.
Uses and Applications
Ethanol finds widespread use as a solvent, antiseptic, and fuel additive. It is a key ingredient in alcoholic beverages and is also used in the production of various chemicals, pharmaceuticals, and cosmetics. The U.S. Environmental Protection Agency (EPA) supports its use as a biofuel.
Phenol serves as a precursor to a vast array of materials and chemicals. It is employed in the production of polymers, such as Bakelite and epoxy resins. Phenol is also used as a disinfectant and in the synthesis of pharmaceuticals, dyes, and herbicides. According to the World Health Organization (WHO), phenol has limited applications in direct consumer products due to its toxicity.
The differences in their reactivity and toxicity dictate their distinct applications.
Safety and Handling
While ethanol is generally considered safe for consumption in diluted forms (alcoholic beverages), it is still a flammable and volatile substance. High concentrations can cause irritation and central nervous system depression. Proper ventilation and storage are essential when handling ethanol.
Phenol is significantly more hazardous than ethanol. It is corrosive and can cause severe burns upon skin contact. Inhalation or ingestion of phenol can lead to serious health problems, including organ damage and even death. Strict safety protocols, including the use of personal protective equipment (PPE), are mandatory when handling phenol.
Material Safety Data Sheets (MSDS) for both compounds provide comprehensive safety information and handling procedures. Always consult these documents before working with either chemical.
Distinguishing Between Ethanol and Phenol
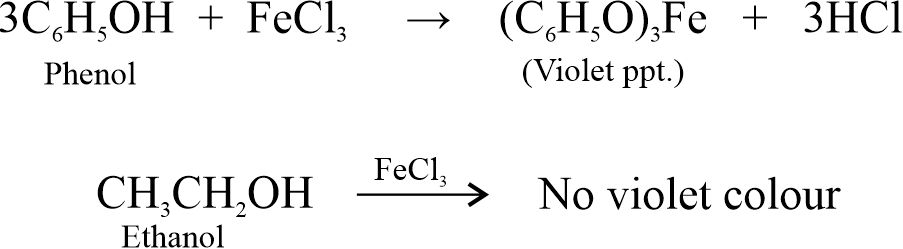
Various chemical tests can easily differentiate between ethanol and phenol. For instance, the ferric chloride test yields a distinct color change with phenol due to the formation of a complex with the iron ions.
Ethanol does not react with ferric chloride under the same conditions. Litmus paper can be used to distinguish between the two: phenol will turn blue litmus paper slightly red, indicating its weak acidity, while ethanol will have no effect.
Spectroscopic techniques, such as NMR and IR spectroscopy, offer definitive methods for identifying and distinguishing between ethanol and phenol based on their unique spectral signatures.
Future Outlook
Research into sustainable production methods for both ethanol and phenol is ongoing. Efforts are focused on developing more environmentally friendly processes that reduce reliance on fossil fuels. Bio-ethanol production, derived from renewable sources, is gaining increasing attention, driven by the need for sustainable fuels and reduced carbon emissions.
Similarly, research is exploring alternative feedstocks for phenol production to reduce its environmental impact and reliance on petroleum-based resources. Green chemistry principles are guiding the development of safer and more sustainable methods for both chemicals.
The accurate identification and handling of ethanol and phenol remain crucial. Enhanced awareness and education are essential to prevent accidental misuse and ensure the safe application of these important chemicals across various industries and research fields. The need to emphasize safety protocols can not be over stated.