Does Epsom Salt Dissolve In Water

Breaking News: Questions surrounding the solubility of Epsom salt have sparked widespread debate online, leaving many confused about its practical applications. Experts confirm that Epsom salt does indeed dissolve in water.
This report clarifies the facts regarding Epsom salt's solubility, addressing concerns and providing essential information for consumers and those in therapeutic fields.
Epsom Salt: Unveiling the Truth
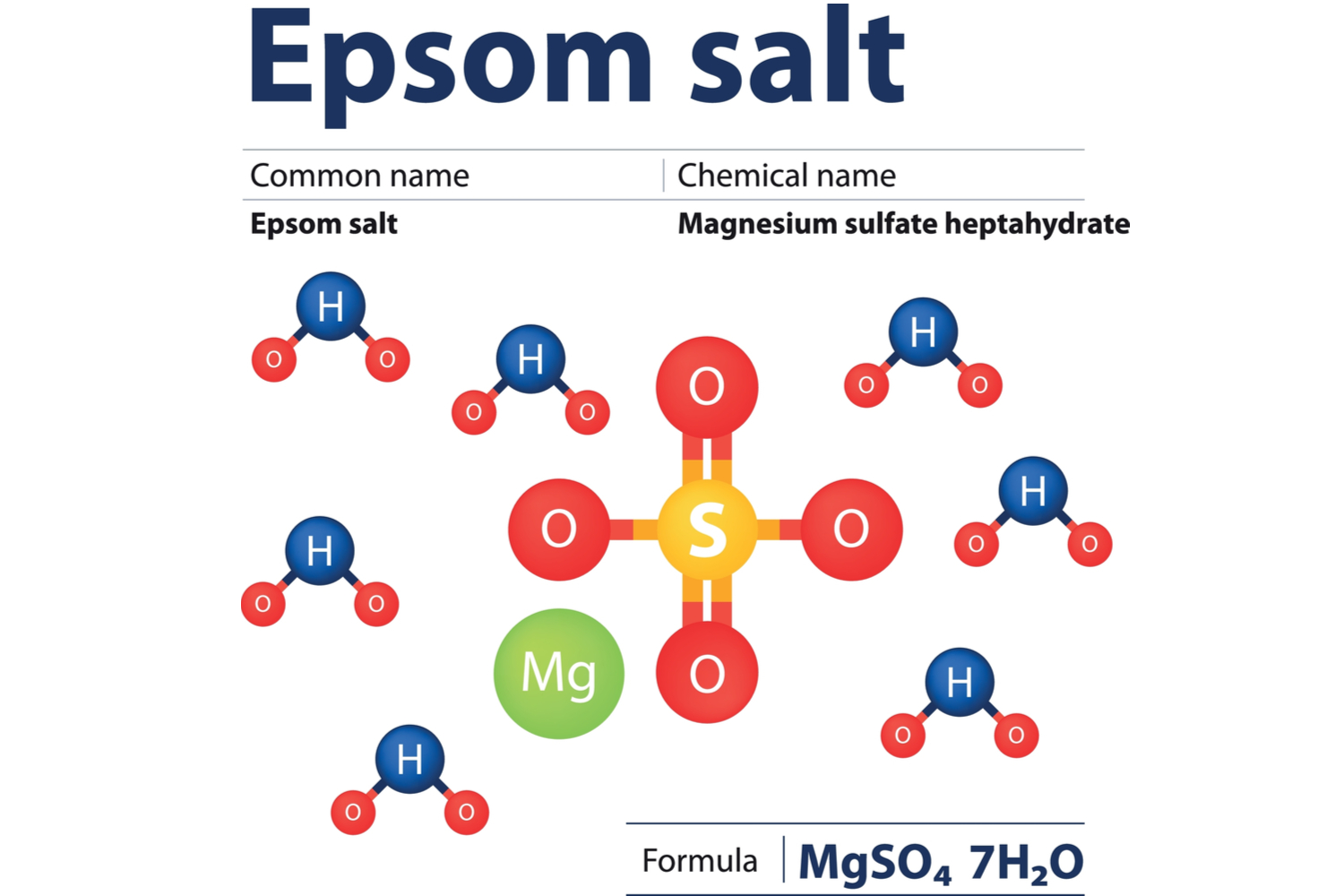
What is Epsom salt? Epsom salt, chemically known as magnesium sulfate (MgSO4), is a naturally occurring mineral compound.
It is often used in baths, foot soaks, and even gardening for its purported health benefits.
The Central Question: Does it Dissolve? The primary question under investigation is whether Epsom salt dissolves in water.
The short answer, according to scientific consensus and practical observation, is a resounding yes.
The Science Behind Solubility
Chemical Properties: The key to Epsom salt's solubility lies in its ionic nature.
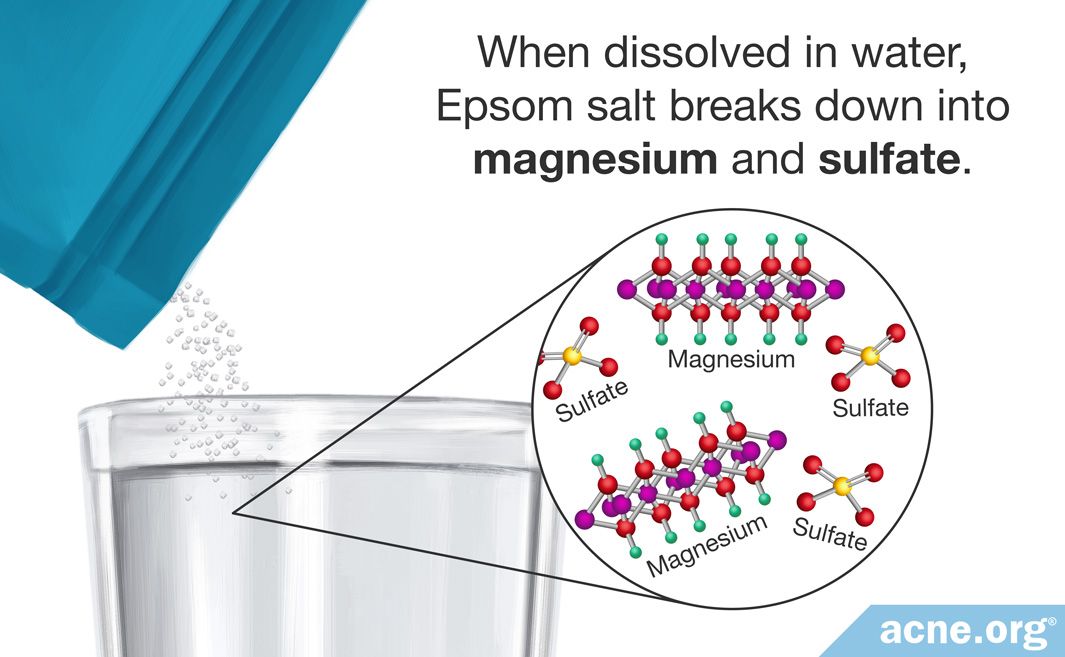
Magnesium sulfate is an ionic compound that readily dissociates into magnesium (Mg2+) and sulfate (SO42-) ions when introduced to water (H2O).
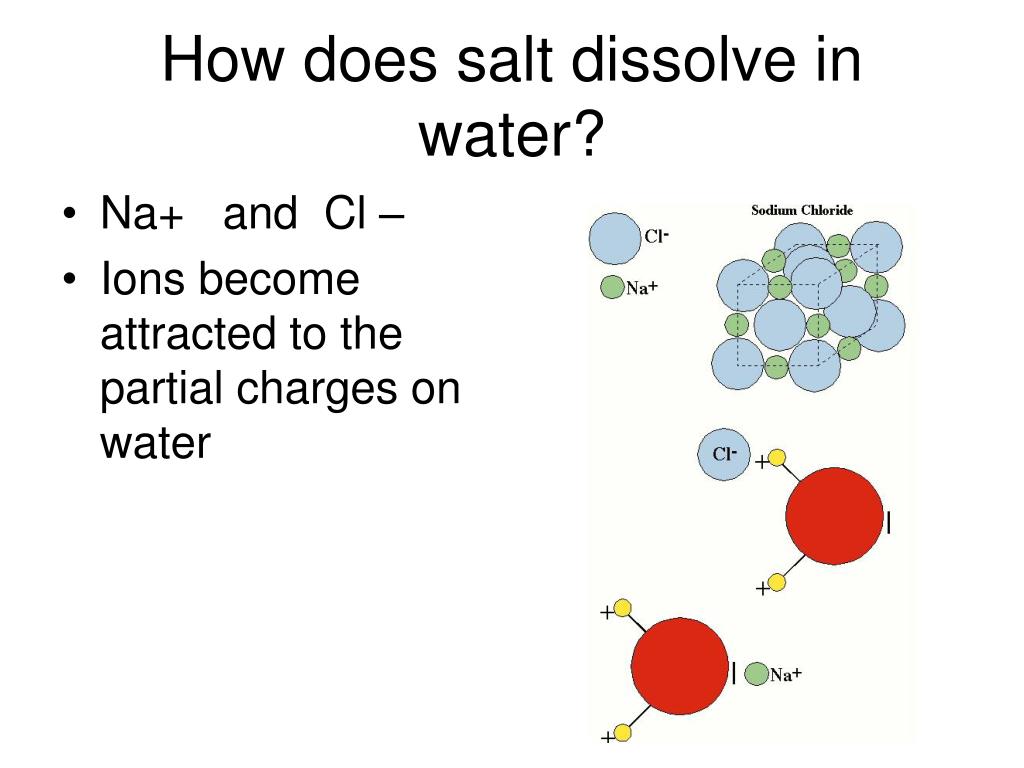
Polarity Matters: Water, being a polar solvent, effectively interacts with these charged ions.
The positively charged magnesium ions are attracted to the partially negative oxygen atoms in water molecules, while the negatively charged sulfate ions are drawn to the partially positive hydrogen atoms.
Hydration Process: This interaction, known as hydration, effectively pulls the ions apart and disperses them throughout the water, resulting in a solution.
Evidence of Dissolution
Laboratory Observations: Controlled experiments consistently demonstrate that Epsom salt dissolves in water.
Increasing water temperature generally increases the rate and extent of dissolution.
Real-World Examples: Anyone who has added Epsom salt to a bath or foot soak can visually observe the crystals disappearing over time, confirming their dissolution.
Factors Affecting Dissolution Rate
Temperature: As mentioned, warmer water facilitates faster dissolution.
Stirring: Agitation or stirring helps to speed up the process by continuously exposing undissolved crystals to fresh solvent.
Concentration: There's a limit to how much Epsom salt can dissolve in a given amount of water.
Beyond this saturation point, the salt will no longer dissolve and will remain as undissolved crystals.
Debunking Misconceptions
Online Confusion: The recent confusion appears to stem from misinterpretations of scientific language and anecdotal reports.
Some users may have confused the time it takes to dissolve with the salt not dissolving at all.
Importance of Reliable Sources: It's crucial to rely on verifiable scientific information and established sources when evaluating scientific claims.
Practical Implications
Bath and Soaks: Knowing that Epsom salt dissolves is critical for its therapeutic use in baths and soaks.
Dissolved magnesium sulfate is believed to be absorbed through the skin, potentially providing relief from muscle aches and inflammation, though scientific evidence for this specific mechanism is still under investigation.
Gardening: In gardening, dissolved Epsom salt can provide magnesium and sulfur to plants, promoting healthy growth.
Conclusion and Next Steps
In summary, Epsom salt does dissolve in water, a fact supported by scientific principles and practical observation.
Further research continues to explore the specific health benefits and optimal usage of Epsom salt.
Consumers are encouraged to consult with healthcare professionals or experts in relevant fields for personalized advice and information.