Evaluate The Medical Aesthetics Company Revance On Dermal Fillers

The pursuit of youthful vitality has fueled a multi-billion dollar medical aesthetics industry, with dermal fillers at its forefront. Among the players vying for dominance is Revance Therapeutics, a company making waves with its innovative approach to neuromodulators and, increasingly, dermal fillers.
But how does Revance measure up in this competitive landscape, particularly when assessing the efficacy, safety, and overall market impact of its dermal filler offerings? This article delves into a comprehensive evaluation of Revance's dermal filler business, analyzing scientific data, expert opinions, and market trends to provide a clear picture of the company's position and prospects.
Revance's Dermal Filler Portfolio: A Closer Look
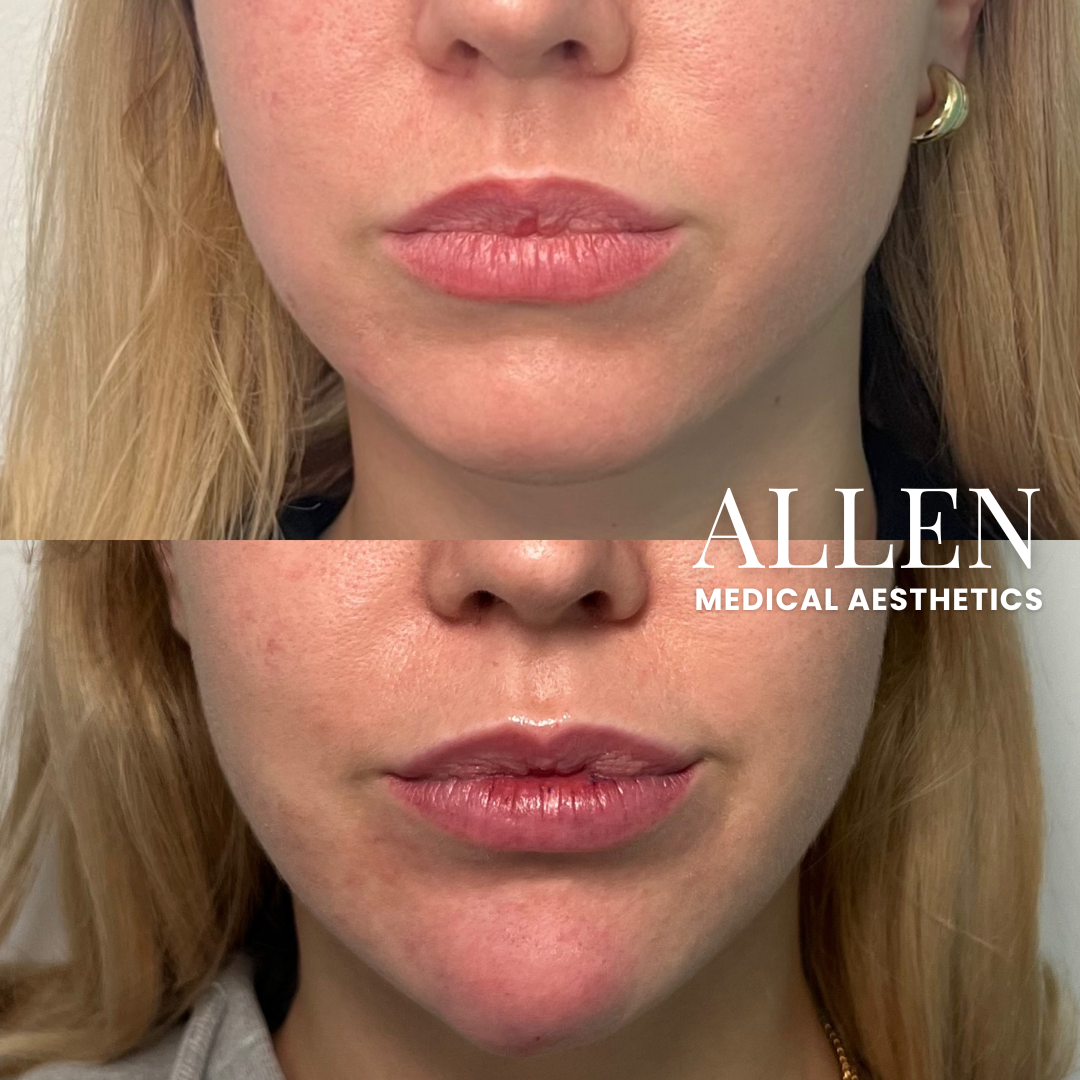
Revance's presence in the dermal filler market is primarily anchored by its RHA® Collection. These fillers are designed with a resilient hyaluronic acid (HA) formulation, aiming to provide natural-looking and long-lasting results.
The RHA® Collection differentiates itself by using a unique HA crosslinking process, which according to Revance, preserves the HA's natural structure, allowing it to more closely mimic the HA found naturally in the skin.
This is meant to result in fillers that can better adapt to facial movements, potentially leading to a more dynamic and less "frozen" appearance.
The Science Behind RHA®: Efficacy and Longevity
Clinical trials have been conducted to assess the efficacy and safety of the RHA® Collection. These studies, often published in peer-reviewed journals, serve as crucial evidence for evaluating the fillers' performance.
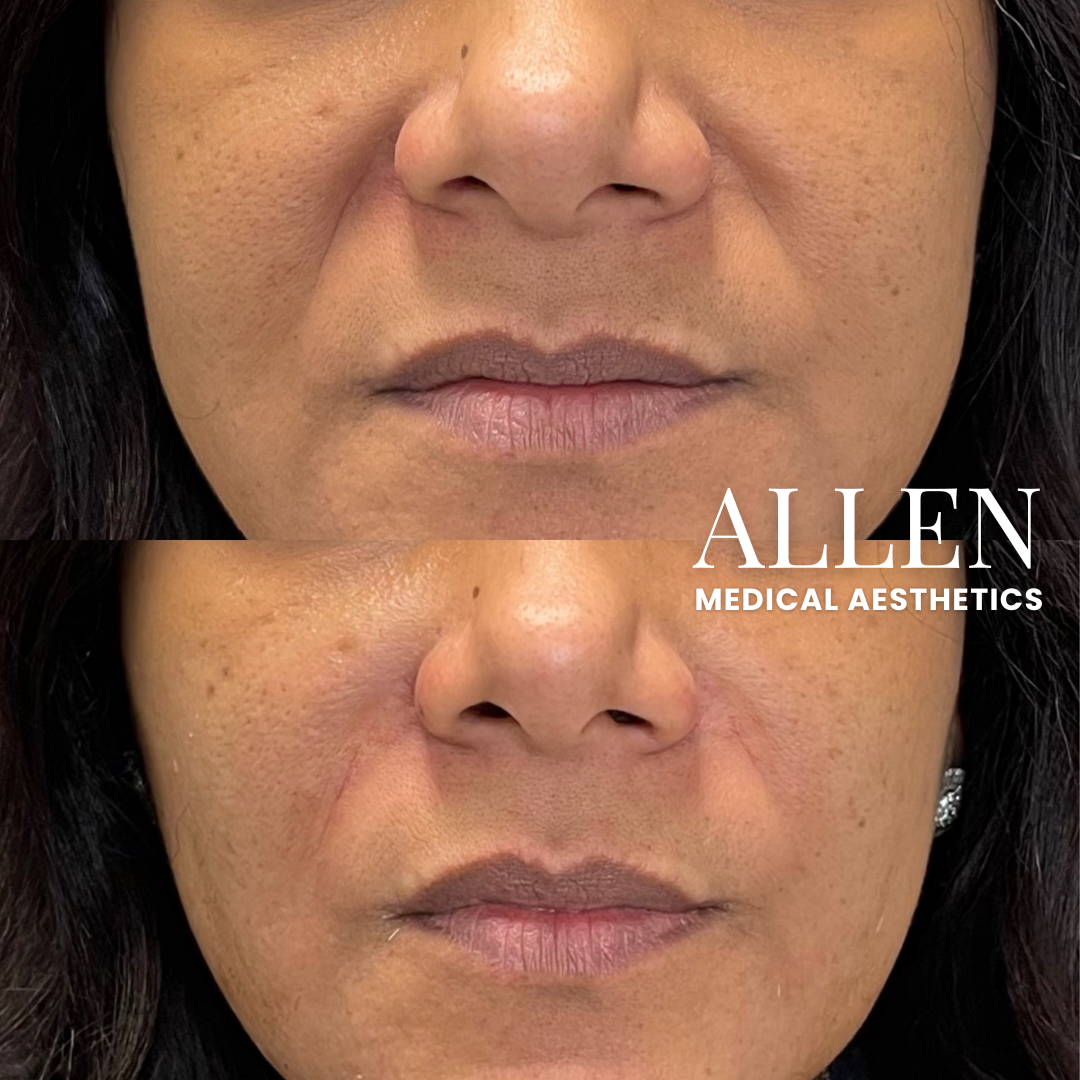
One pivotal study, for example, investigated the use of RHA® fillers for correcting moderate to severe nasolabial folds (NLFs). The study, reported in the *Aesthetic Surgery Journal*, showed a statistically significant improvement in NLF severity as assessed by both investigators and subjects.
Data presented at medical conferences and in company publications also suggest that the RHA® Collection offers a competitive duration of effect, with some formulations demonstrating visible results lasting up to 15 months or longer in some patients.
Safety Profile and Adverse Events
Safety is paramount in the medical aesthetics field, and dermal fillers are no exception. Revance has conducted extensive safety evaluations of the RHA® Collection, and the results are generally positive.
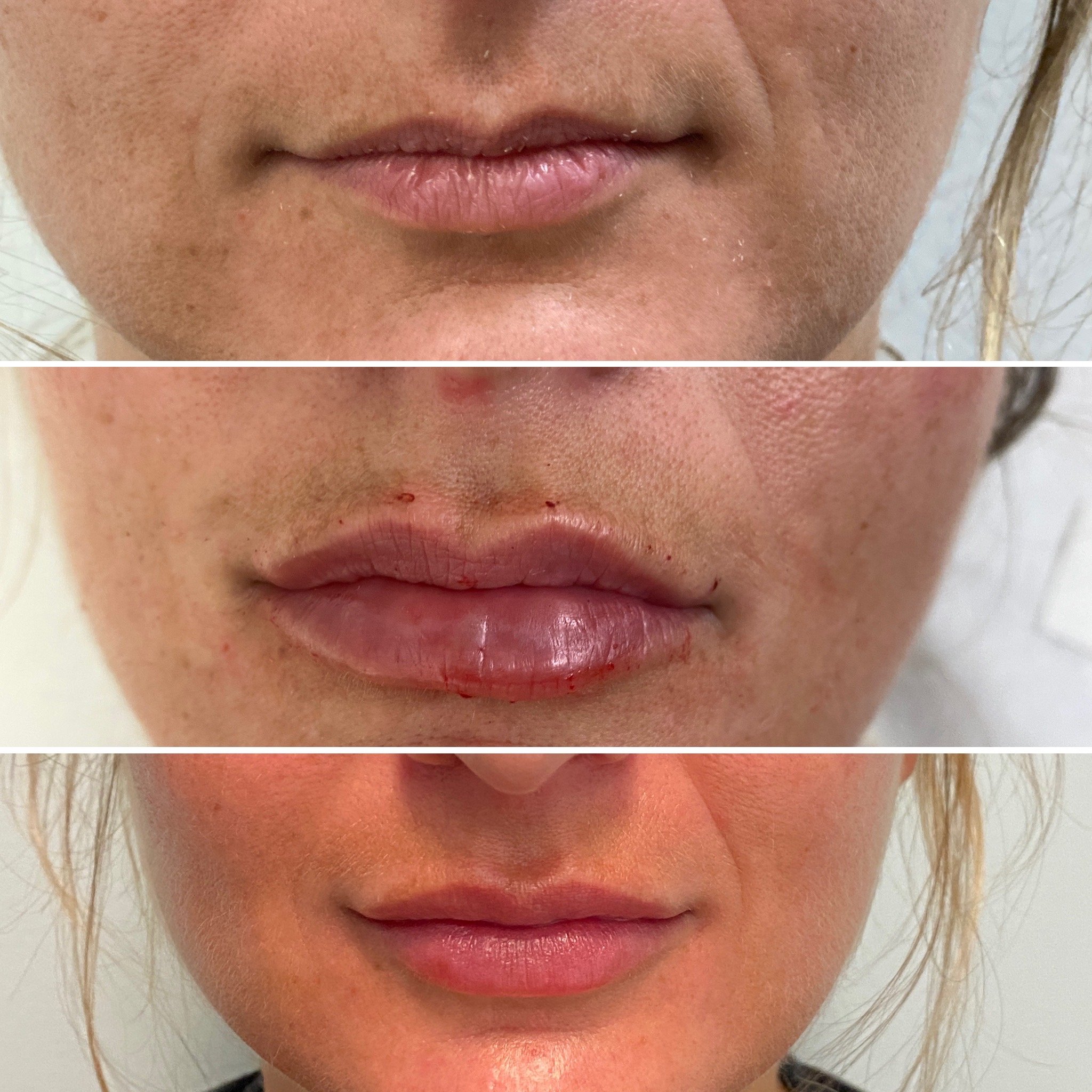
Common side effects associated with RHA® fillers are typical of HA fillers in general and include injection-site reactions such as swelling, bruising, redness, and tenderness. These reactions are typically mild to moderate and resolve within a few days.
Serious adverse events, such as vascular occlusion or infection, are rare but possible with any dermal filler injection. Revance provides training and resources for healthcare providers to minimize these risks.
Market Position and Competitive Landscape
The dermal filler market is dominated by established players like Allergan (AbbVie), Galderma, and Merz Aesthetics. Revance, while a relative newcomer to this specific segment, is leveraging its reputation in the broader aesthetics field, particularly its challenge to Botox with its Daxxify neuromodulator.
Revance's strategy involves positioning the RHA® Collection as a premium offering, emphasizing its unique formulation and potential for natural-looking results. This strategy aims to attract both practitioners and patients seeking a higher-quality experience.
However, gaining market share in this competitive landscape requires significant investment in marketing, sales, and education. Revance's success depends on effectively communicating the value proposition of RHA® and building strong relationships with key opinion leaders (KOLs) and aesthetic practices.
Expert Opinions and Practitioner Adoption
The adoption of new dermal fillers by practitioners is heavily influenced by expert opinions and real-world clinical experience. Many dermatologists and plastic surgeons are increasingly using the RHA® Collection, sharing their experiences at conferences and in publications.
These early adopters often highlight the fillers' ease of use, the natural-looking results they achieve, and the positive feedback they receive from patients. Their endorsements play a critical role in driving wider adoption.
However, some practitioners may prefer the familiarity and predictability of more established brands. Revance needs to continue building trust and confidence within the medical community to expand its market presence.
The Future of Revance's Dermal Filler Business
Looking ahead, Revance has several opportunities to further strengthen its dermal filler business. Continued investment in research and development could lead to new formulations with even better efficacy, longevity, or safety profiles.
Expanding the RHA® Collection to address a wider range of aesthetic concerns, such as lip augmentation or tear trough correction, could also broaden the company's market reach. Strategic partnerships and collaborations could provide access to new distribution channels and technologies.
Ultimately, Revance's success in the dermal filler market will depend on its ability to consistently deliver high-quality products, build strong relationships with practitioners, and effectively communicate the value proposition of its offerings to patients.
Challenges and Risks
Despite the promising outlook, Revance faces certain challenges and risks. The dermal filler market is highly competitive, and established players have significant resources and brand recognition.
Regulatory hurdles and potential safety concerns could also impact the company's growth. Maintaining a strong focus on product quality and safety is essential for mitigating these risks.
Furthermore, economic downturns or shifts in consumer preferences could affect demand for aesthetic treatments in general, potentially impacting Revance's sales.
Conclusion: A Promising Contender
Revance is emerging as a promising contender in the dermal filler market with its RHA® Collection. The company's focus on developing innovative HA formulations and its commitment to clinical research are noteworthy.
While Revance faces competition from established players, its strategic approach and positive reception from practitioners suggest a bright future. Its success hinges on continued innovation, effective marketing, and a steadfast dedication to patient safety.
The medical aesthetics field is ever-evolving, and Revance's ongoing efforts will determine its ultimate place in this dynamic industry. Whether Revance can truly disrupt the market remains to be seen, but the initial signs are compelling.





.JPG)