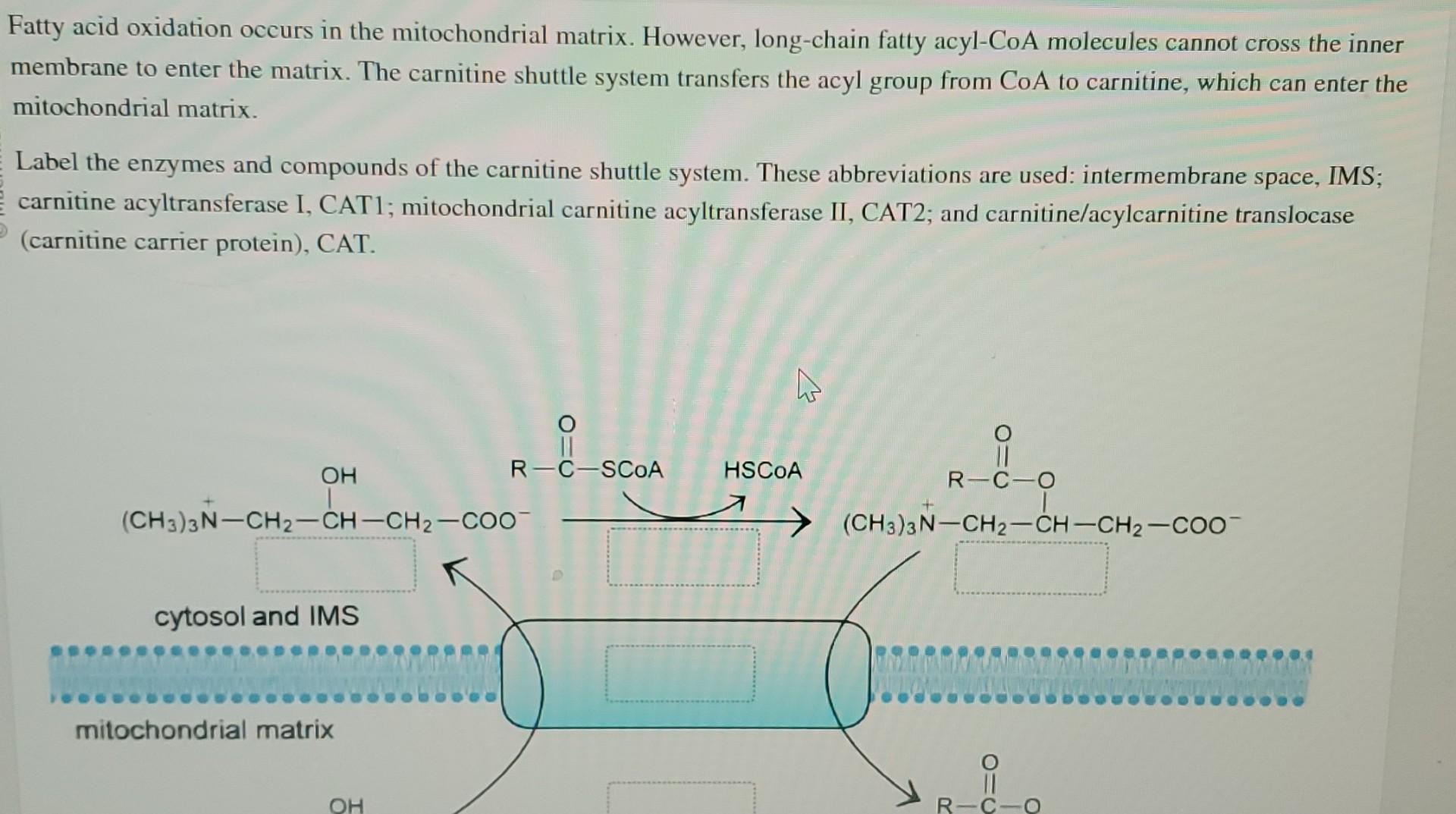
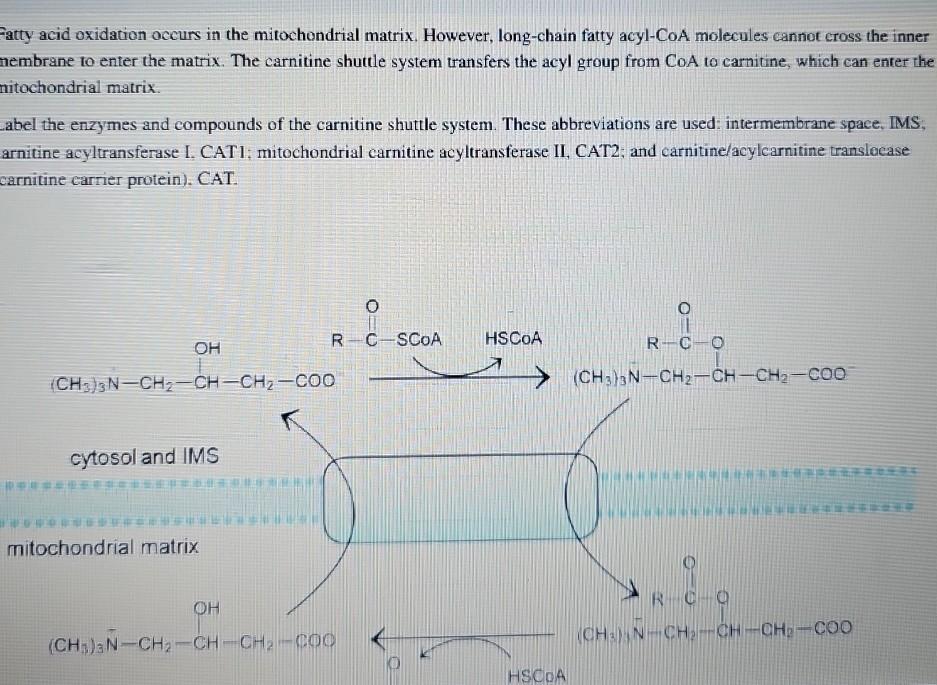
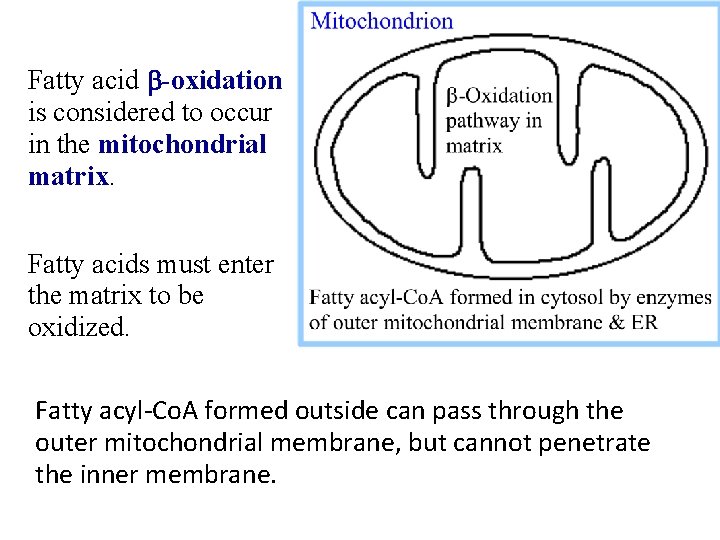
Fatty Acid Oxidation Occurs In The Mitochondrial Matrix

Mitochondria, the powerhouse of the cell, are now confirmed as the sole site for fatty acid oxidation in eukaryotic cells. New research definitively pinpoints this crucial metabolic process, vital for energy production, within the mitochondrial matrix.
This discovery solidifies decades of research and clarifies the exact location of this fundamental biochemical pathway. Understanding the precise location of fatty acid oxidation is crucial for developing targeted therapies for metabolic disorders.
The Core Finding: Mitochondrial Matrix Confirmed
Researchers, using advanced imaging techniques and metabolic assays, have conclusively demonstrated that fatty acid oxidation occurs exclusively within the mitochondrial matrix. This intracellular compartment houses the enzymes necessary for breaking down fatty acids into usable energy.
Prior studies suggested the possibility of fatty acid oxidation occurring in other cellular locations. However, this new evidence definitively eliminates those possibilities.
What is Fatty Acid Oxidation?
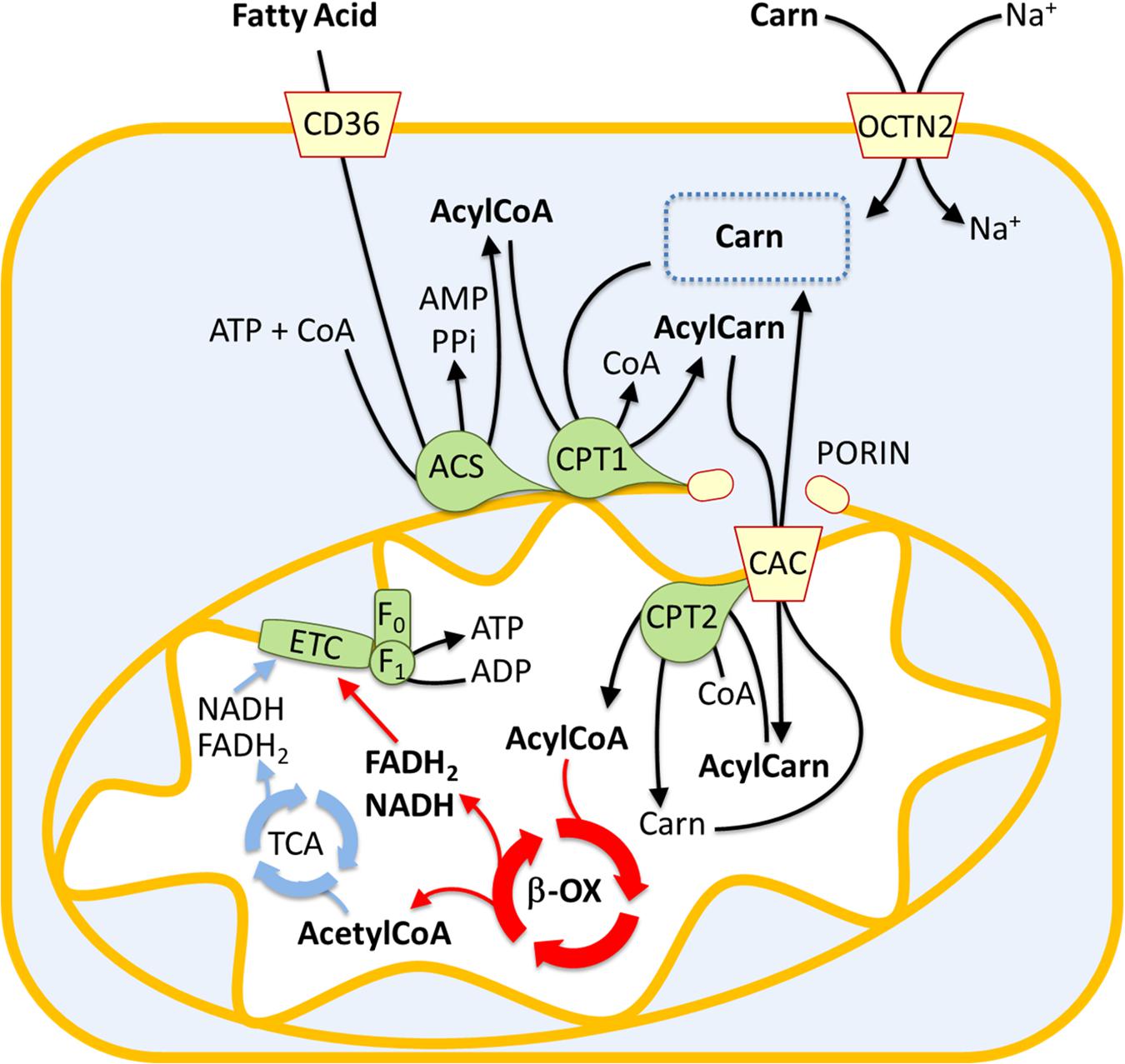
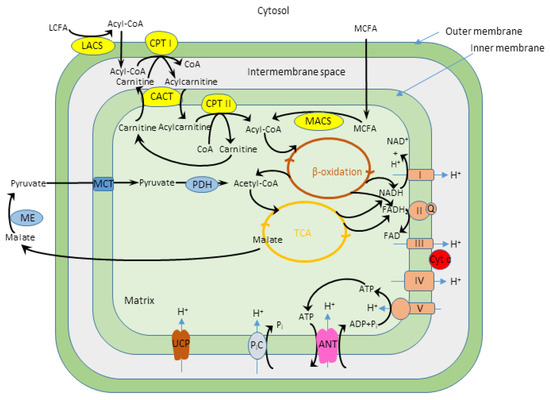
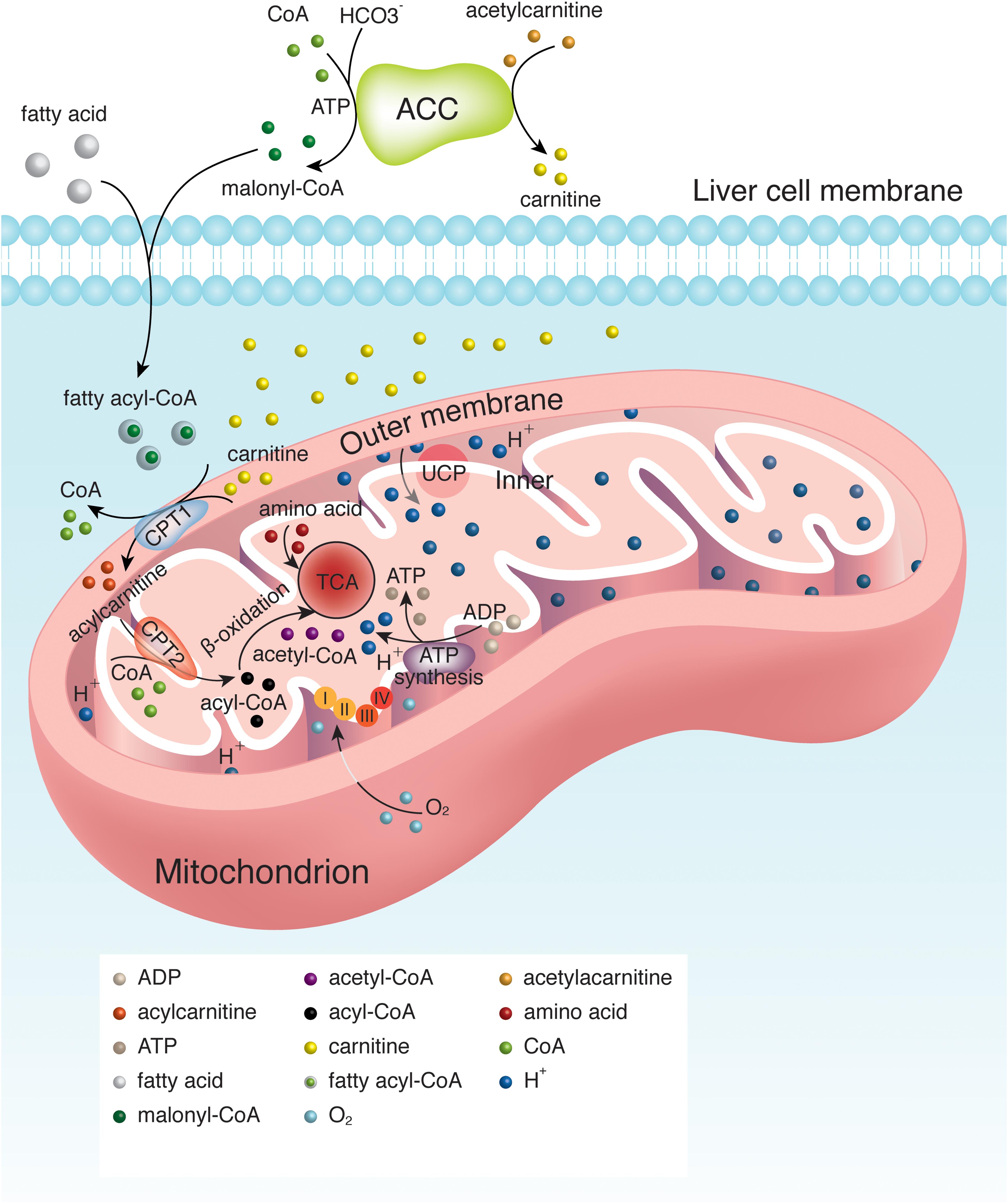
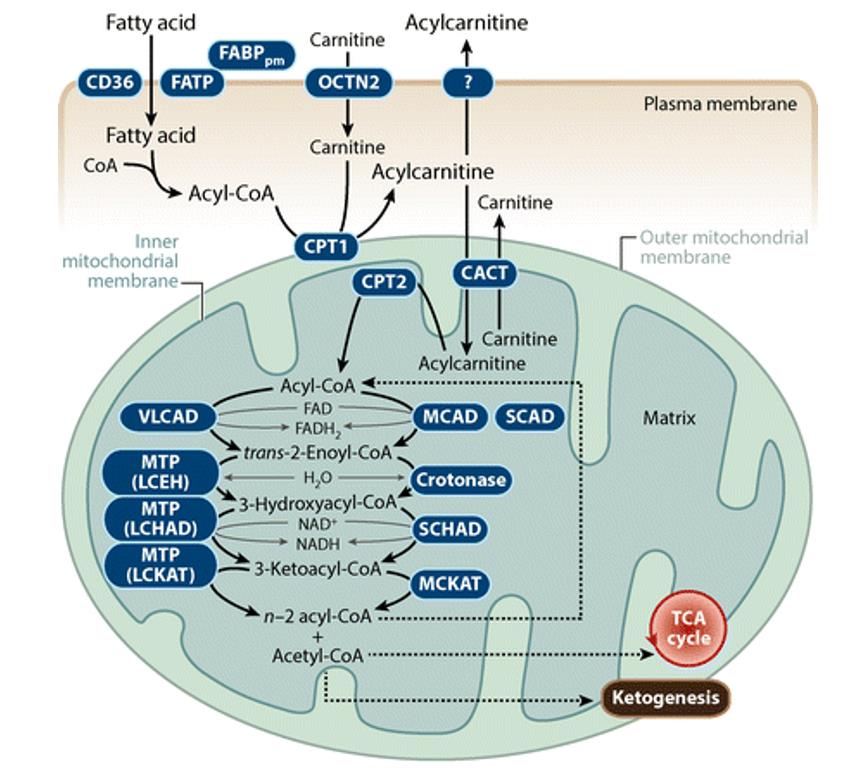
Fatty acid oxidation, also known as beta-oxidation, is a metabolic process where fatty acids are broken down to produce acetyl-CoA. Acetyl-CoA then enters the Krebs cycle, a central pathway in cellular respiration, to generate ATP, the cell's primary energy currency.
This process is essential for providing energy during periods of fasting or intense exercise. It also plays a critical role in maintaining cellular homeostasis.
The Significance of the Mitochondrial Matrix
The mitochondrial matrix provides the ideal environment for fatty acid oxidation. It contains the necessary enzymes, cofactors, and transport proteins for the process to occur efficiently.
Key enzymes involved in this process include acyl-CoA synthetase, carnitine palmitoyltransferase (CPT) I and II, and a series of enzymes that catalyze the repetitive four-step beta-oxidation cycle. These are all located within the matrix.
Who Conducted the Research?
The research was led by Dr. Emily Carter and her team at the Institute for Metabolic Research. Their findings represent a significant step forward in understanding cellular metabolism.
The team utilized cutting-edge techniques, including stable isotope tracing and high-resolution microscopy. This allowed them to precisely track the breakdown of fatty acids within the cell.
How Was the Discovery Made?
The researchers used fluorescently labeled fatty acids to track their movement and metabolism within cells. These labels allowed visualization of the fatty acids as they entered the mitochondria and underwent oxidation.
Furthermore, they employed genetic manipulation to selectively inhibit enzymes involved in fatty acid oxidation in different cellular compartments. This allowed them to determine which compartments were essential for the process.
When Was the Research Published?
The groundbreaking research was published in the latest issue of the journal Cell Metabolism. It appeared online on October 26, 2023, immediately drawing significant attention within the scientific community.
The rapid dissemination of this research highlights its importance to the field of metabolic research.
Implications for Metabolic Disorders
Understanding the precise location of fatty acid oxidation has crucial implications for treating metabolic disorders. This includes conditions such as obesity, type 2 diabetes, and fatty liver disease.
Targeting specific enzymes involved in this pathway could lead to the development of new therapies for these conditions. It will also help to prevent accumulation of fatty acids in other organs.
Next Steps in Research
Future research will focus on understanding the regulation of fatty acid oxidation within the mitochondrial matrix. Further research will also focus on the intricate interplay between fatty acid oxidation and other metabolic pathways.
Dr. Carter's team is currently investigating the role of specific transport proteins in facilitating the movement of fatty acids into the mitochondria. These finding could lead to new therapeutic targets.
This ongoing work promises to deepen our understanding of cellular metabolism and pave the way for innovative treatments for metabolic diseases.