For Most Substances Solubility Is Blank As Temperature

Imagine a steaming mug of tea on a chilly winter morning. As you stir in a spoonful of sugar, it disappears almost instantly into the warmth. Now, picture the same scenario with a glass of iced tea. You might notice those sugar crystals lingering at the bottom, stubbornly refusing to dissolve. This simple observation hints at a fundamental principle that governs much of the natural world: the relationship between temperature and solubility.
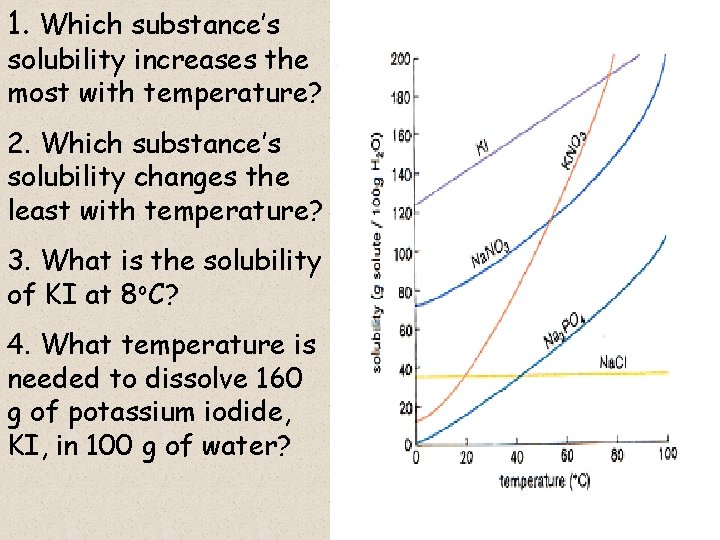
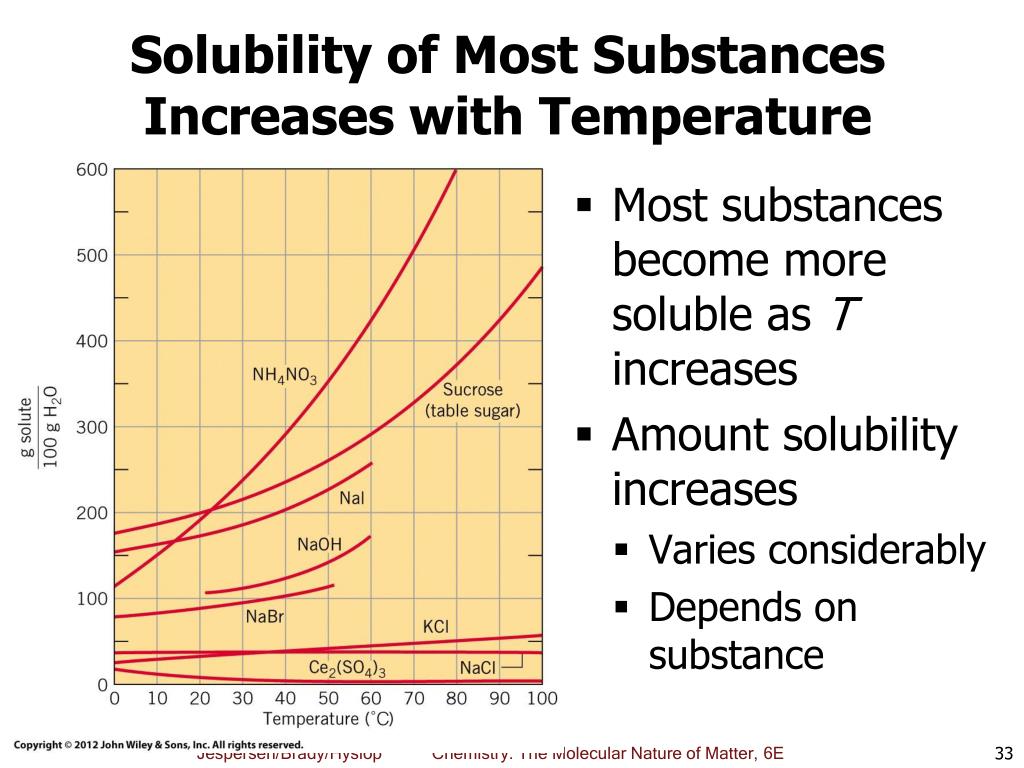
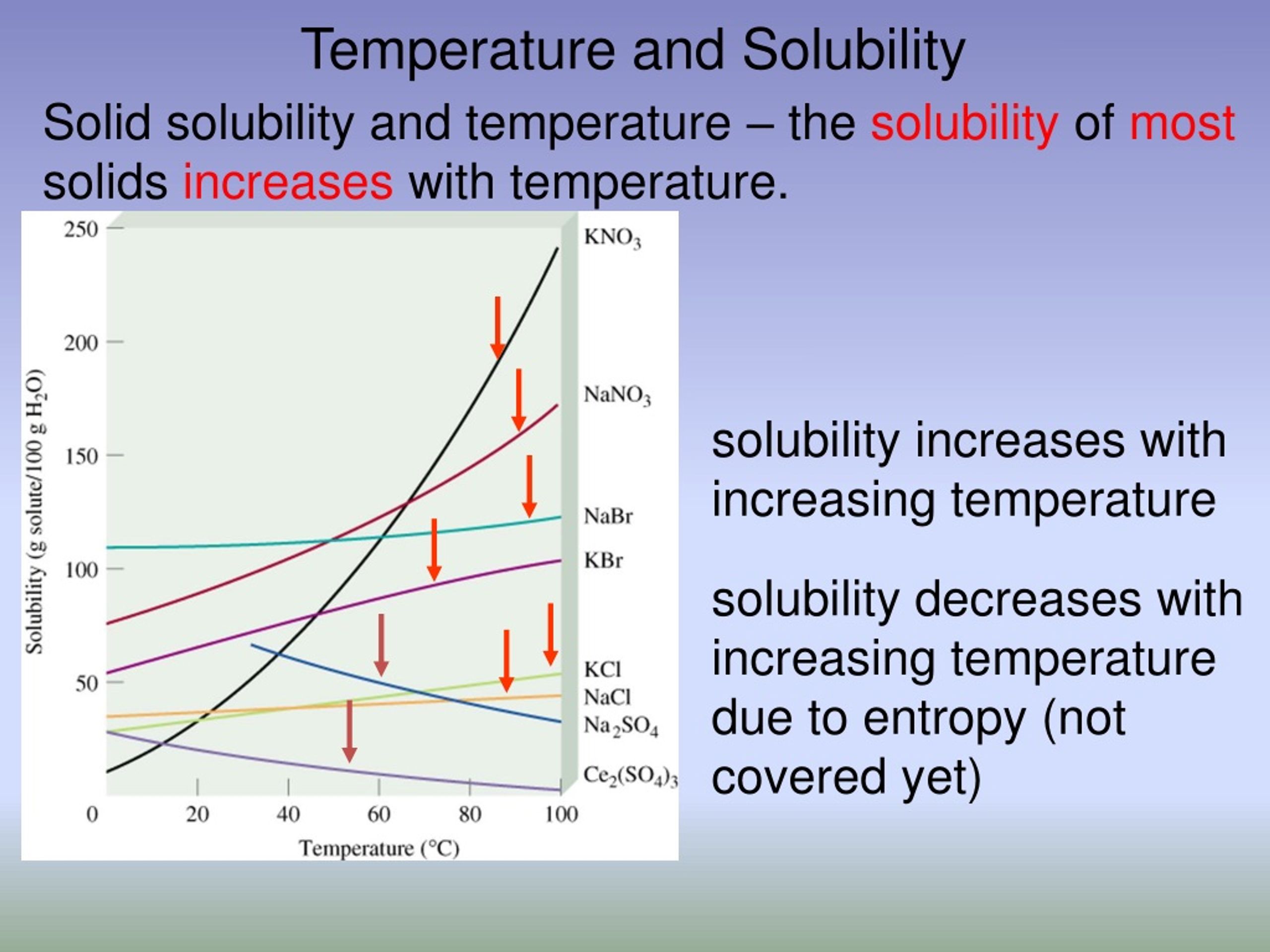
At its core, the relationship between temperature and solubility is simple: For most substances, solubility increases as temperature increases. This phenomenon, while seemingly straightforward, has profound implications across various fields, from chemistry and biology to everyday cooking and industrial processes. Understanding this relationship is crucial for optimizing reactions, designing new materials, and even preparing the perfect cup of coffee.
The Basics of Solubility
Solubility, in its simplest form, refers to the maximum amount of a solute that can dissolve in a given amount of solvent at a specific temperature. The solute is the substance being dissolved (like sugar), and the solvent is the substance doing the dissolving (like water). When a substance dissolves, its molecules become dispersed amongst the molecules of the solvent, forming a homogeneous mixture called a solution.
This process isn't always easy, and it involves overcoming intermolecular forces. Solutes and solvents interact through various forces, such as Van der Waals forces, dipole-dipole interactions, and hydrogen bonding. For a solute to dissolve, the attractive forces between the solute and solvent molecules must be strong enough to overcome the attractive forces within the solute itself.
Temperature's Role in Solubility
Increasing the temperature of a solvent generally increases the kinetic energy of its molecules. This heightened energy allows solvent molecules to more effectively break apart the intermolecular forces holding the solute together. Consequently, more solute molecules can disperse throughout the solvent, leading to a higher solubility.
This effect is particularly pronounced for solids dissolving in liquids. The higher temperature provides the energy needed to break the bonds holding the solid lattice together. The increased kinetic energy also helps the solvent molecules to surround and stabilize the individual solute particles.
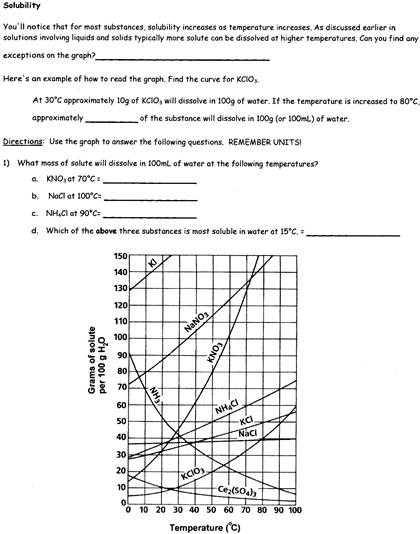
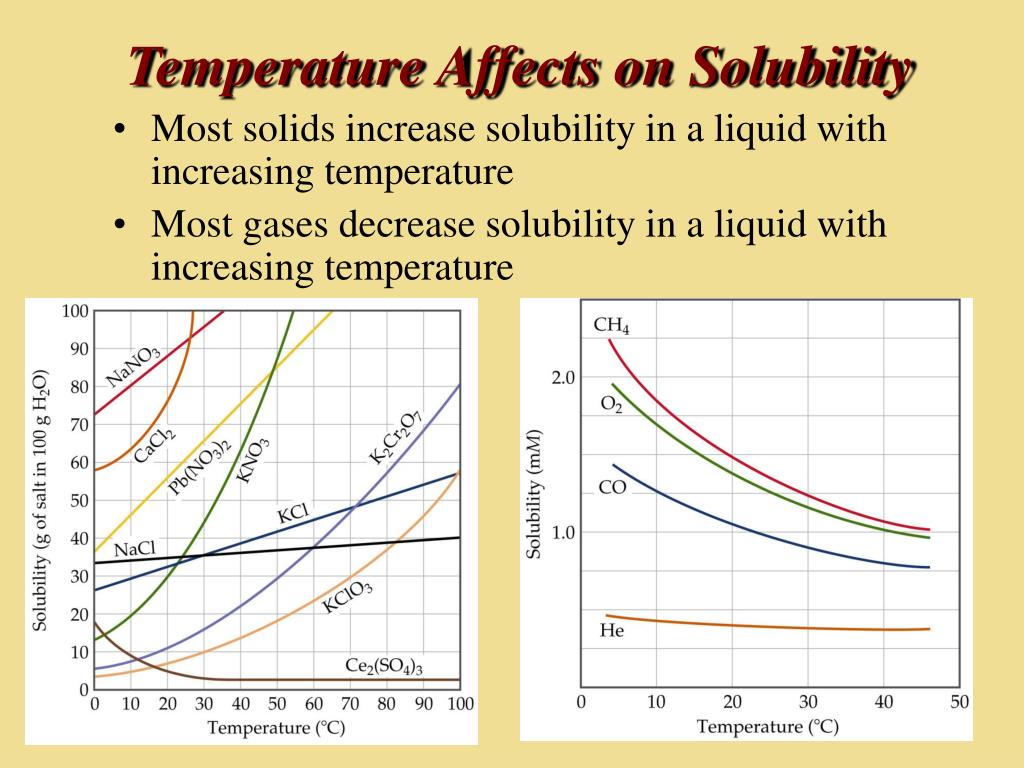
However, there are exceptions to this rule. The solubility of gases in liquids tends to decrease as temperature increases. Think of a carbonated beverage going flat as it warms up; the carbon dioxide gas escapes the liquid more readily at higher temperatures.
Why Gases Behave Differently
The contrasting behavior of gases stems from the nature of their interactions with liquids. When a gas dissolves in a liquid, it occupies spaces between the liquid molecules.
Increasing the temperature provides the gas molecules with more kinetic energy, allowing them to overcome the attractive forces holding them in solution and escape back into the gaseous phase. This is why boiling water can effectively remove dissolved gases, a common practice in laboratories and industries.
Applications Across Disciplines
The temperature-solubility relationship is not just a textbook concept; it is a powerful tool with applications in numerous fields. In chemistry, it's used to control crystallization processes. By carefully adjusting the temperature, chemists can selectively precipitate out desired compounds, purifying them from mixtures.
In the pharmaceutical industry, solubility plays a critical role in drug delivery. Many drugs are administered as solutions, and their solubility directly affects their absorption and bioavailability in the body. Understanding the temperature dependence of drug solubility is essential for formulating effective medications.
The food industry also relies heavily on this principle. The production of jams and jellies involves dissolving large amounts of sugar in water, a process that is significantly enhanced by heating. The crafting of candies and chocolates also hinges on precise temperature control to achieve the desired texture and consistency.
Environmental Significance
The solubility of gases in water has significant environmental implications, especially in aquatic ecosystems. The amount of dissolved oxygen in water, crucial for the survival of fish and other aquatic organisms, decreases as water temperature increases. This phenomenon can lead to oxygen depletion in warmer waters, harming aquatic life.
Furthermore, the solubility of pollutants in water is affected by temperature. Understanding these relationships is vital for predicting the fate and transport of contaminants in aquatic environments and for developing effective remediation strategies. Climate change, with its associated rise in global temperatures, poses a significant threat to water quality and aquatic ecosystems due to these solubility effects, according to a 2021 IPCC report.
Everyday Examples
Even in our daily lives, we encounter this principle frequently. Making a cup of coffee or tea, as mentioned earlier, relies on the increased solubility of coffee grounds or tea leaves in hot water to extract the flavorful compounds. When brewing iced tea or coffee, a concentrated hot brew is often made first, then chilled to prevent the beverage from becoming weak or flavorless.
Home brewing also utilizes the solubility-temperature relationship in the mashing process, where grains are steeped in hot water to extract sugars. The temperature is carefully controlled to optimize the extraction of desired compounds while minimizing the extraction of undesirable ones.
A Look Ahead
As scientific knowledge advances, researchers continue to explore the nuances of the temperature-solubility relationship. New computational models and experimental techniques are providing deeper insights into the complex interactions between solutes and solvents at the molecular level. These advances are paving the way for designing new materials with tailored properties and developing more efficient industrial processes.
Moreover, a better understanding of these principles is crucial for addressing environmental challenges, such as water pollution and climate change. By leveraging this knowledge, scientists and engineers can develop innovative solutions to protect our planet and ensure a sustainable future.
The next time you stir sugar into your tea or coffee, remember the fundamental principle at play: for most substances, solubility increases as temperature increases. This seemingly simple observation connects us to a vast network of scientific understanding that touches almost every aspect of our world, from the smallest molecular interactions to the grandest environmental processes. It’s a reminder that even the most ordinary occurrences can reveal extraordinary truths about the nature of things.









+of+water+over+a+range+of+temperatures..jpg)