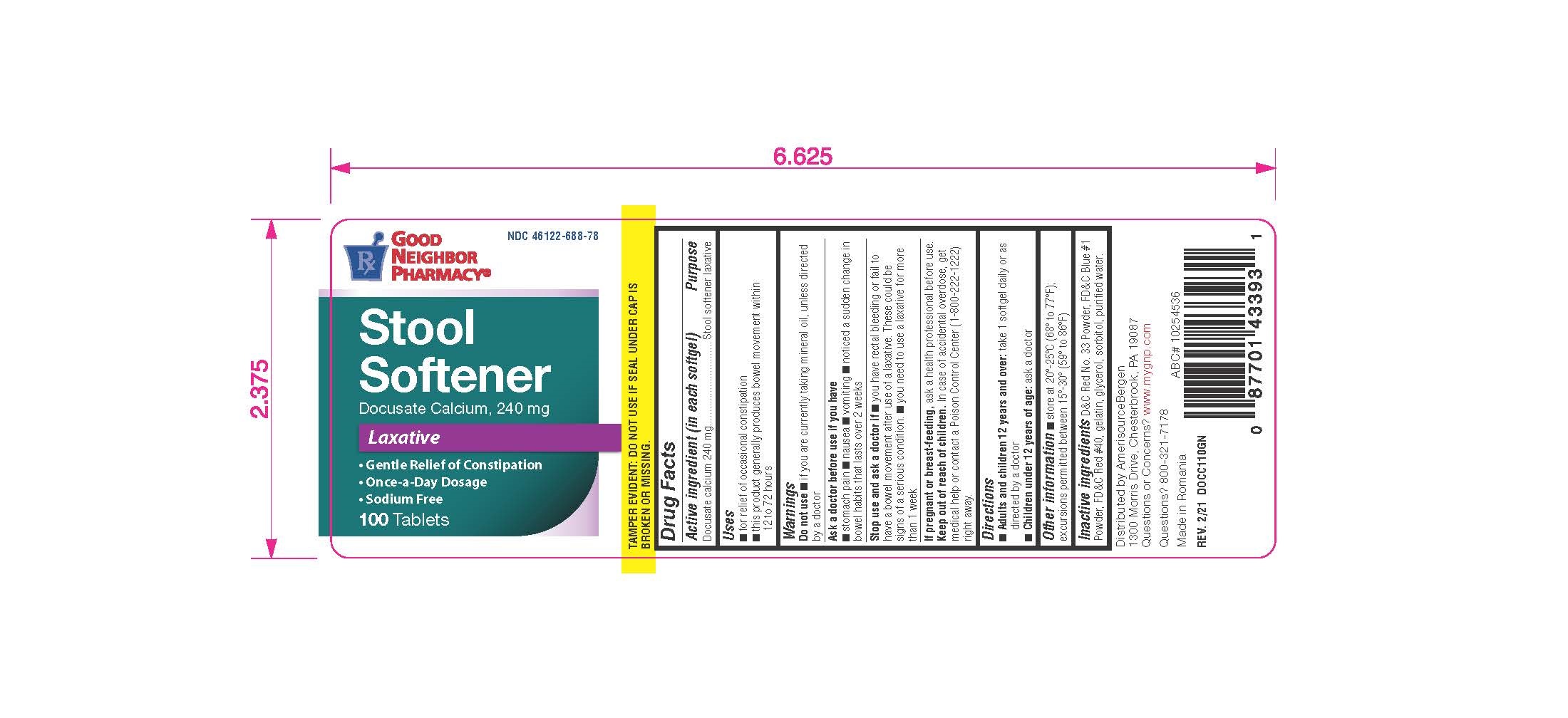
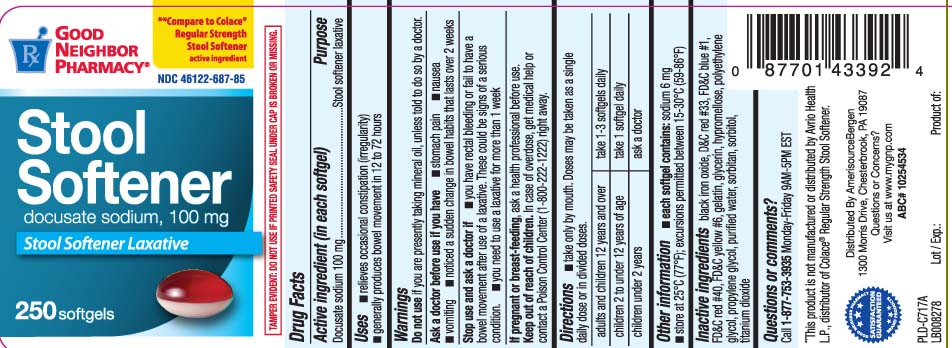
Good Neighbor Pharmacy Stool Softener

URGENT: A nationwide recall has been issued for Good Neighbor Pharmacy Stool Softener due to potential contamination. Consumers are urged to immediately check their medicine cabinets and cease use of the affected product.
This recall, initiated by L Perrigo Company, raises serious concerns about product safety and poses a potential health risk to users, particularly those with compromised immune systems.
Immediate Action Required
The Good Neighbor Pharmacy Stool Softener, distributed across the United States, is being recalled after laboratory testing revealed contamination.
The specific contaminant has been identified as Burkholderia cepacia complex (Bcc), a group of bacteria that poses a significant risk, particularly to individuals with cystic fibrosis, weakened immune systems, and chronic lung diseases.
Who is affected? The recall impacts anyone who purchased Good Neighbor Pharmacy Stool Softener. Special attention should be given to those with underlying health conditions.
Identifying the Recalled Product
What is being recalled? The recall involves all lots of Good Neighbor Pharmacy Stool Softener sold in bottles of 100-count softgels.
Check your bottle for the Good Neighbor Pharmacy brand. Verify the 100-count softgel format to confirm it is part of the recall.
Where was the product sold? Good Neighbor Pharmacy stores nationwide distributed the affected stool softener.
When did the distribution occur? The exact timeframe is not explicitly stated, but the recall encompasses all lots currently in circulation.
Health Risks and Symptoms
Infection with Bcc can cause a range of symptoms, from mild respiratory infections to more serious conditions like pneumonia.
Individuals with weakened immune systems are at a higher risk of severe complications. Look out for fever, cough, congestion, and shortness of breath.
Anyone experiencing these symptoms after taking Good Neighbor Pharmacy Stool Softener should seek immediate medical attention.
How to Respond to the Recall
How should consumers respond? Immediately discontinue use of the recalled stool softener.
Return the product to the place of purchase for a full refund. Contact your physician or healthcare provider if you have any concerns.
Dispose of the product properly to prevent accidental ingestion by others.
L Perrigo Company's Response
L Perrigo Company is working with the FDA to manage the recall effectively. They are notifying distributors and retailers to remove the product from shelves.
The company has established a dedicated phone line and website for consumers to obtain more information. Direct inquiries to 1-800-xxx-xxxx. Visit [website address - fictitious].
This voluntary recall reflects the company's commitment to product safety. L Perrigo Company aims to minimize disruption and safeguard consumers’ health.
Ongoing Developments
The FDA is closely monitoring the situation and will provide updates as new information becomes available. Check the FDA website regularly for official announcements.
Further investigation is underway to determine the source of the contamination. The recall remains in effect until further notice.
The public is urged to remain vigilant and report any adverse reactions to the FDA's MedWatch program. This helps ensure the safety of all consumers.