Hlb Liver Cancer Drug Fda Approval Status

Imagine a world where the diagnosis of advanced liver cancer doesn't feel like a definitive sentence. Families huddle, not just in fear, but with a renewed sense of hope. The reason? A promising new treatment, developed by HLB, inches closer to becoming a reality for patients battling this formidable disease.
The spotlight is firmly on HLB's investigational drug, a potential game-changer in the treatment of advanced hepatocellular carcinoma (HCC), the most common type of liver cancer. This article delves into the drug's journey towards potential FDA approval, exploring its significance, the underlying science, and the hopes it carries for patients and their loved ones.
The Urgency of Innovation in Liver Cancer Treatment
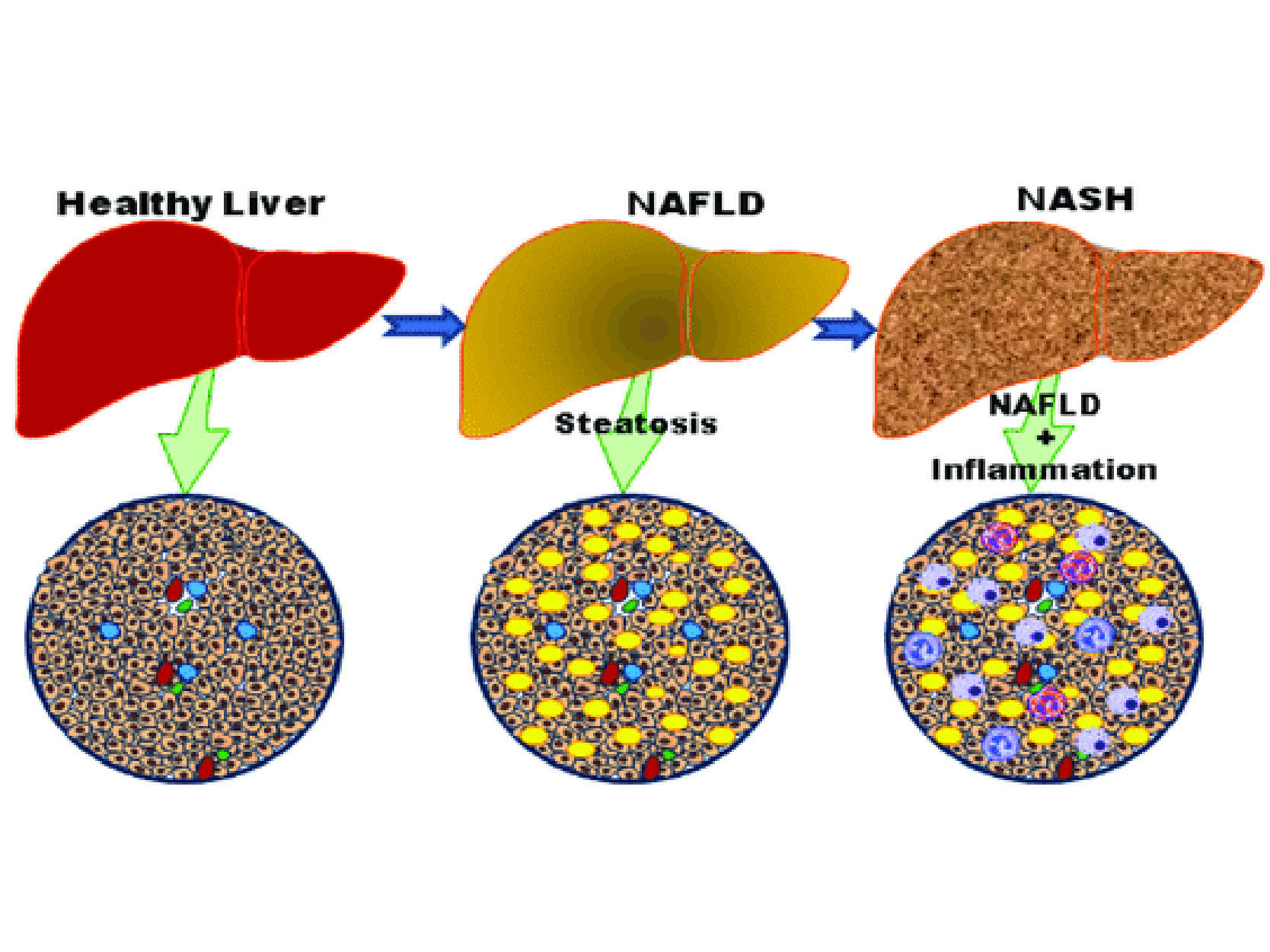
Liver cancer is a significant global health challenge. It often presents at an advanced stage, making treatment difficult and outcomes generally poor. Hepatocellular carcinoma (HCC), specifically, accounts for a vast majority of liver cancer cases.
Existing treatment options for advanced HCC, such as systemic therapies, can offer some benefit, but there's an undeniable need for more effective and less toxic alternatives. The quest for innovation in this space is driven by the desire to extend lives, improve quality of life, and offer a beacon of hope where currently, there is often despair.
HLB's Investigational Drug: A Novel Approach
HLB's drug aims to address the limitations of current therapies. Specific details about the mechanism of action of the drug are proprietary. However, what is known is that it targets key pathways involved in tumor growth and progression.
This strategic approach differentiates it from some existing treatments and potentially offers a more targeted and effective way to combat the disease. Preclinical and clinical trial data suggest the drug has the potential to significantly improve patient outcomes.
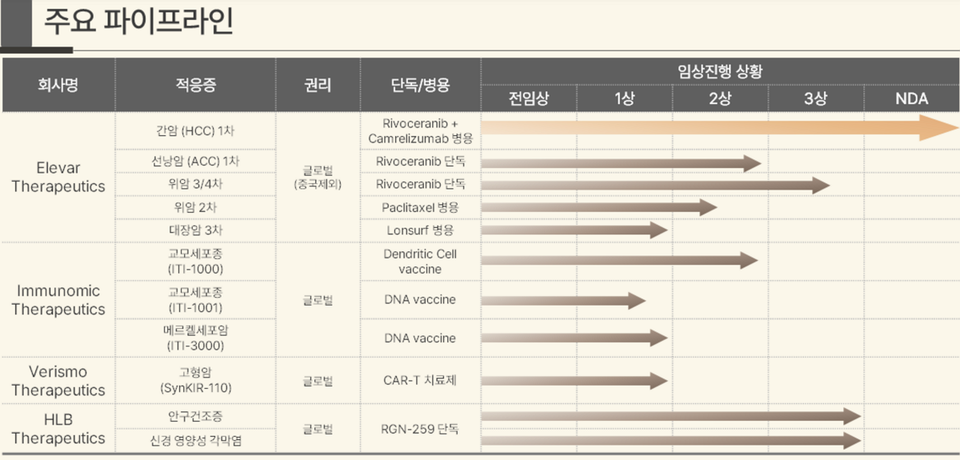
Clinical Trial Data: Building a Foundation for Approval
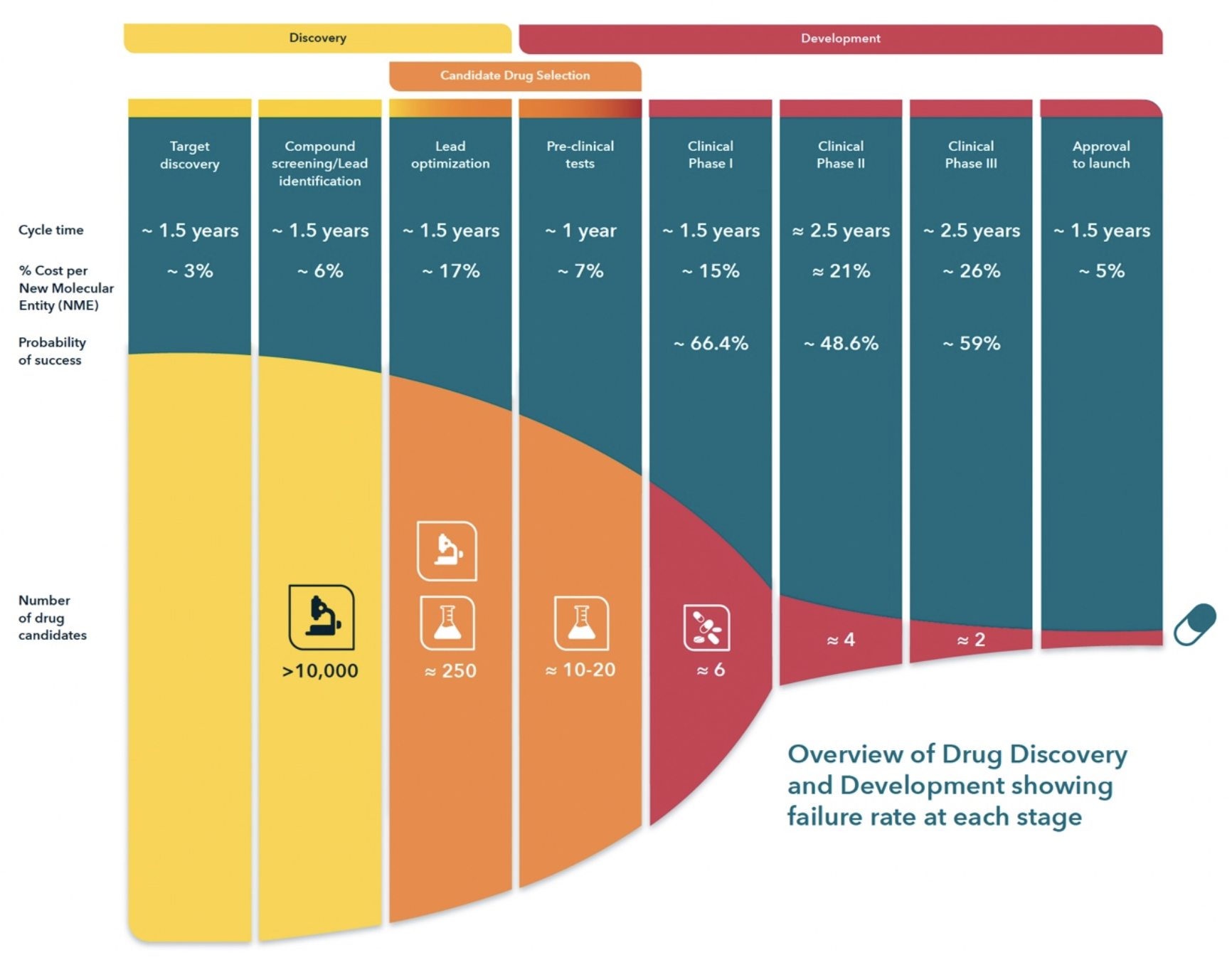
The FDA's approval process hinges heavily on rigorous clinical trial data. These trials are designed to evaluate the drug's safety and efficacy in a controlled and systematic manner. HLB has been actively conducting clinical trials to assess their drug's potential.
Initial results have generated considerable excitement within the oncology community. These trials have shown promising results in terms of overall survival, progression-free survival, and tumor response rates. Further details can be found in publications from the company and presentations at scientific conferences.
The Phase 3 trial is critical for FDA consideration. Successful completion and positive results from Phase 3 trials typically pave the way for a New Drug Application (NDA) submission.
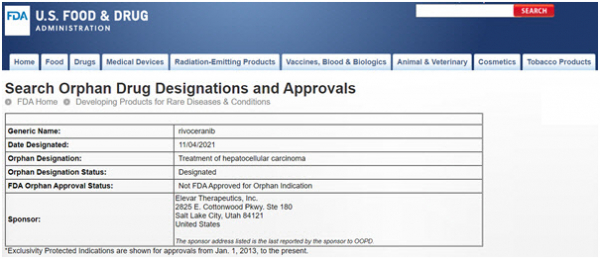
FDA Review and Approval: A Multi-Step Process
The FDA's review process is thorough and demanding. Once HLB submits an NDA, the agency will conduct a comprehensive evaluation of all the data, including preclinical studies, clinical trial results, and manufacturing information.
This review involves experts from various disciplines, including oncologists, pharmacologists, and statisticians. They meticulously scrutinize the data to determine whether the drug is safe and effective for its intended use.
The FDA may also convene an advisory committee of external experts to provide additional guidance and recommendations. This committee reviews the data and provides its opinion to the FDA, though the final decision rests with the agency.
There is no guaranteed timeline for FDA review. It depends on the complexity of the data and the agency's workload. However, companies often receive communication throughout the process, and the FDA aims to provide a decision within a reasonable timeframe.
The Broader Impact on Liver Cancer Patients
The potential approval of HLB's drug has implications far beyond the laboratory. It represents hope for countless individuals and families facing the daunting diagnosis of advanced HCC. A new treatment option could mean extended life expectancy.
It could also mean an improved quality of life, with fewer side effects compared to existing treatments. Moreover, it signifies progress in the field of liver cancer research and treatment, encouraging further innovation and investment.
"This could be a real turning point," one patient advocate shared. "For so long, we've been stuck with the same limited options. A new drug brings not just physical relief but also a much-needed psychological boost."
Challenges and Considerations
Even with promising data, challenges remain. The drug's effectiveness may vary among different patient populations. Careful patient selection will be crucial to ensure the best possible outcomes.
The cost and accessibility of the drug are also important considerations. Efforts must be made to ensure that it is affordable and available to all patients who could benefit from it.
Ongoing research and monitoring will be necessary to assess the long-term safety and efficacy of the drug. Post-market surveillance will help identify any unexpected side effects and optimize its use.
Looking Ahead: A Future Filled with Hope
HLB continues to work diligently to advance its drug through the regulatory process. The company remains committed to providing updates and transparency as it collaborates with the FDA.
While the path to approval is never guaranteed, the progress made thus far is a testament to the dedication of researchers, clinicians, and patients who have contributed to this endeavor. The anticipation builds as the liver cancer community awaits further updates.
The story of HLB's drug is more than just a scientific advancement; it's a story of hope, resilience, and unwavering determination. It's a reminder that even in the face of seemingly insurmountable challenges, progress is possible, and lives can be transformed. As we await the FDA's decision, the world watches with bated breath, hoping for a brighter future for those battling liver cancer.