How Long Can Urine Stay Fresh For A Drug Test
The stakes are high. A drug test can determine employment prospects, impact legal proceedings, and even affect athletic careers. The accuracy of these tests hinges, in part, on a critical factor: the integrity of the urine sample. How long does urine truly remain "fresh" enough for a reliable drug test, and what conditions influence its stability?
The window of viability for a urine sample intended for drug testing is surprisingly delicate. This article delves into the factors affecting urine sample stability, exploring the scientifically recommended storage durations, the potential for degradation that can compromise test results, and the practical implications for both individuals and the organizations that rely on drug testing. Understanding these nuances is crucial for ensuring fair and accurate results in a system with significant consequences.
Factors Affecting Urine Stability
Several factors influence how long urine remains suitable for drug testing. These include temperature, light exposure, and the presence of preservatives. Degradation can occur rapidly under unfavorable conditions.
Temperature plays a pivotal role. Elevated temperatures accelerate the breakdown of certain drugs and metabolites in urine. Conversely, refrigeration or freezing can significantly extend the sample's lifespan.
Temperature and Storage Duration
According to guidelines from the Substance Abuse and Mental Health Services Administration (SAMHSA), urine samples should ideally be analyzed as soon as possible after collection. However, this is not always feasible. Refrigeration at 2-8°C (36-46°F) is generally recommended for short-term storage.
SAMHSA guidelines typically suggest that refrigerated urine samples can be viable for approximately 24-72 hours. Beyond this timeframe, the risk of degradation increases, potentially affecting the accuracy of the test results.
For longer storage periods, freezing is the preferred method. Freezing at -20°C (-4°F) or lower can preserve the integrity of the sample for several weeks or even months, though repeated freeze-thaw cycles should be avoided.
The Role of Preservatives
Preservatives are often added to urine samples to inhibit bacterial growth and slow down degradation processes. Common preservatives include boric acid and sodium fluoride. These substances can extend the acceptable storage duration.
The type and concentration of preservative used can vary depending on the specific drugs being tested for. It's crucial to follow the manufacturer's instructions regarding preservative use to ensure accurate results.
However, even with preservatives, the long-term stability of urine samples is not guaranteed. Degradation can still occur over time, particularly for certain drugs and metabolites.
Impact of Degradation on Test Results
Degradation of urine samples can lead to both false-negative and false-positive results. This can have serious consequences for individuals undergoing drug testing. Understanding the degradation process is essential.
False-negative results occur when a drug or metabolite is present in the urine but is not detected due to degradation. This can happen if the drug has broken down into other compounds or if its concentration has fallen below the detection threshold.
False-positive results, while less common, can occur if degradation products interfere with the testing process. These products may mimic the chemical structure of certain drugs, leading to an inaccurate positive result.
Specific Drug Stability
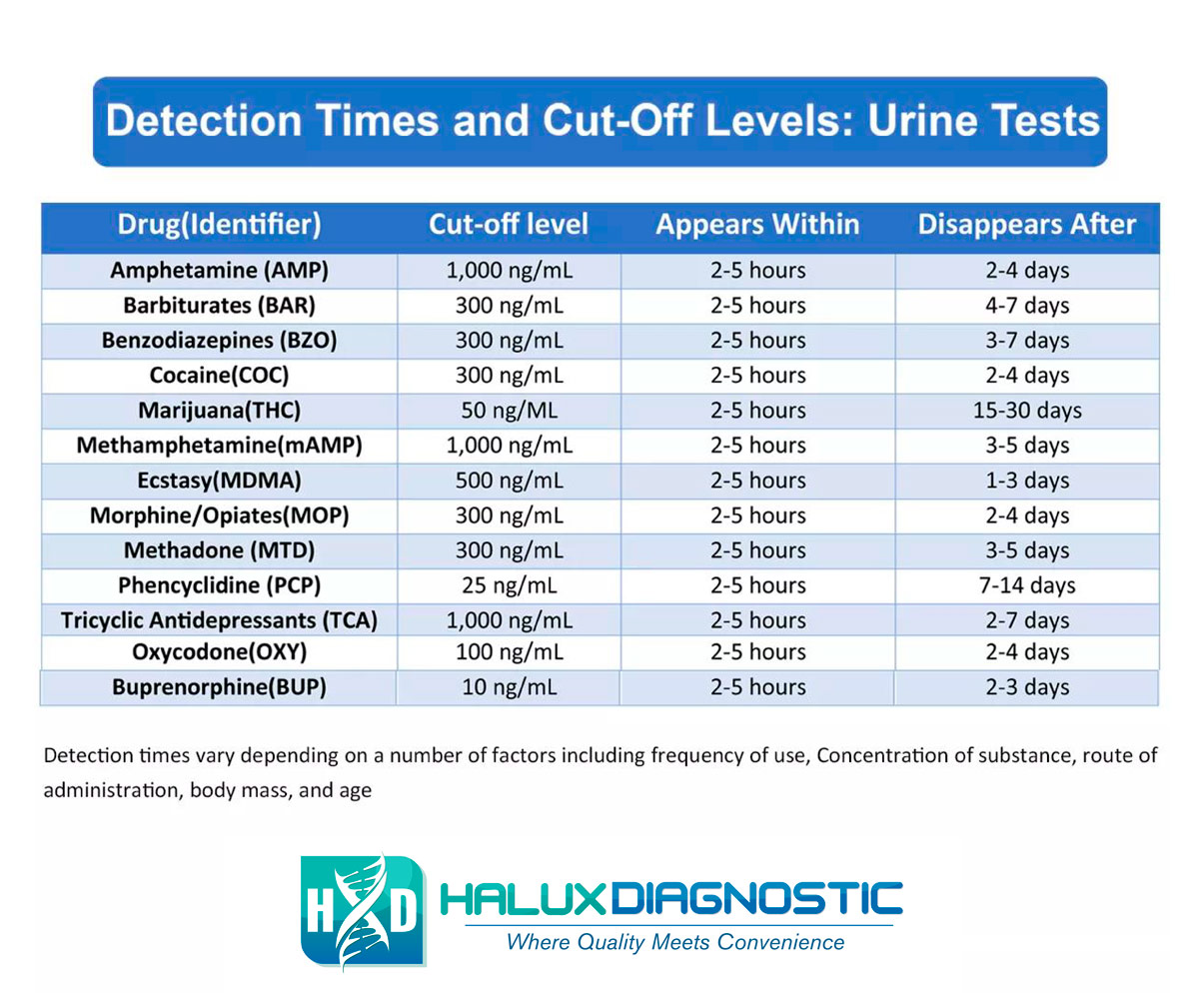
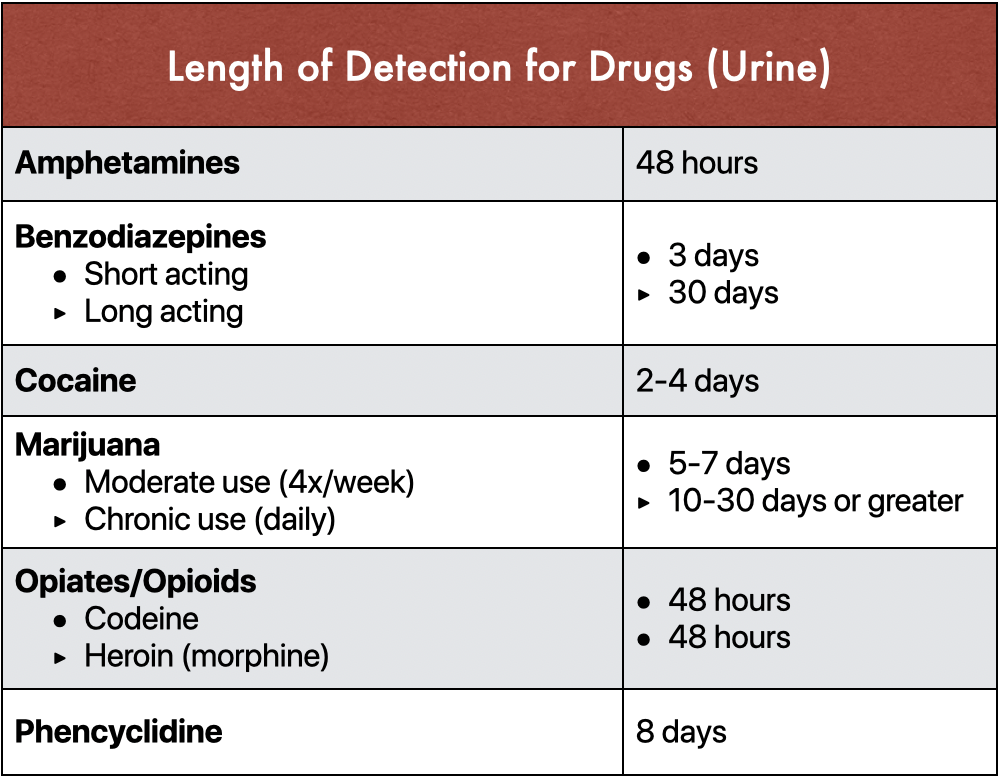
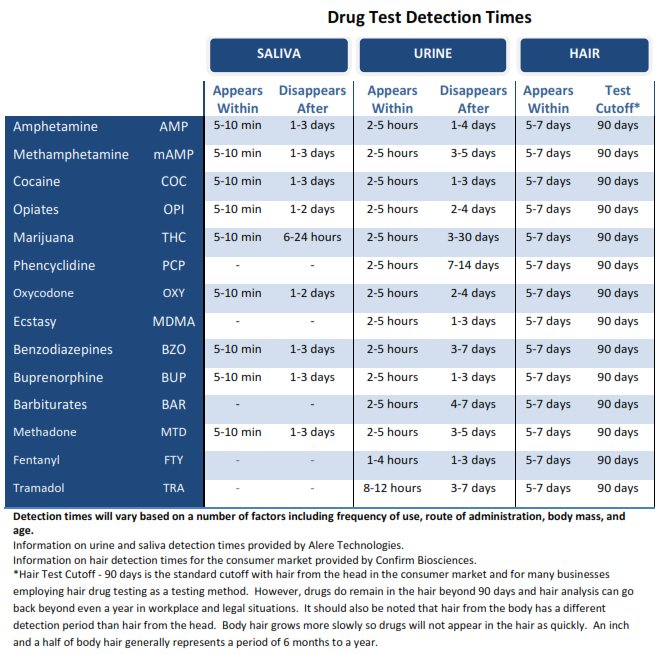
The stability of different drugs and metabolites in urine varies considerably. Some substances are more prone to degradation than others. Tetrahydrocannabinol (THC) metabolites, for example, are relatively stable in urine, particularly when stored properly.
Opioids, on the other hand, can be more susceptible to degradation, especially at room temperature. This is why prompt refrigeration or freezing is crucial for urine samples being tested for opioid use.
The specific degradation pathways and rates for different drugs are complex and depend on various factors. Laboratory analysis and validation studies are necessary to determine the acceptable storage duration for each substance.
Practical Implications and Recommendations
The limited shelf life of urine samples has significant implications for drug testing programs. Laboratories and collection sites must adhere to strict protocols to ensure the integrity of samples. Proper handling and storage are crucial.
Accurate record-keeping is essential. The date and time of collection should be clearly documented, as well as the storage conditions. This information is crucial for assessing the viability of the sample.
Regular quality control measures are necessary to monitor the stability of urine samples. This may involve analyzing control samples with known concentrations of drugs to detect any degradation.
"Laboratories should have documented procedures for the handling, storage, and transportation of urine samples," stresses Dr. Emily Carter, a leading expert in forensic toxicology. "These procedures should be based on scientific evidence and regularly reviewed to ensure their effectiveness."
Individuals undergoing drug testing should also be aware of their rights and responsibilities. They should ensure that the collection process is conducted properly and that the sample is handled according to established protocols.
The Future of Urine Drug Testing
Research is ongoing to develop more stable and reliable methods for urine drug testing. This includes the development of new preservatives and analytical techniques. Scientists are working to enhance the accuracy and efficiency of the testing process.
Point-of-care testing devices are also becoming increasingly popular. These devices allow for rapid on-site analysis of urine samples, reducing the need for long-term storage. This can minimize the risk of degradation and improve the turnaround time for results.
While urine drug testing remains a valuable tool, it is essential to acknowledge its limitations. Proper sample handling and storage are critical for ensuring the accuracy and reliability of results. Continuous research and development are necessary to improve the technology and minimize the risk of errors. Understanding the nuances of urine stability is crucial for maintaining fairness and integrity in drug testing programs.