How Much Atp Does Electron Transport Chain Produce
Imagine a microscopic dance floor, bustling with activity. Tiny molecules waltz with precision, passing electrons like precious gifts. This energetic ballet happens within our cells, powering life itself, and it's all about making ATP—the energy currency of life.
The electron transport chain (ETC), a crucial part of cellular respiration, generates the vast majority of ATP. While textbooks often cite figures like 32 or 34 ATP molecules per glucose, the actual yield is a bit more complex and debated.
Understanding the Electron Transport Chain
To grasp the ATP production, we need to understand the ETC's context. It’s the final stage of cellular respiration, following glycolysis and the Krebs cycle.
These earlier stages break down glucose, a simple sugar, and produce electron carriers. NADH and FADH2 are these carriers. They ferry electrons to the ETC, setting the stage for the ATP-generating finale.
The Players and the Process
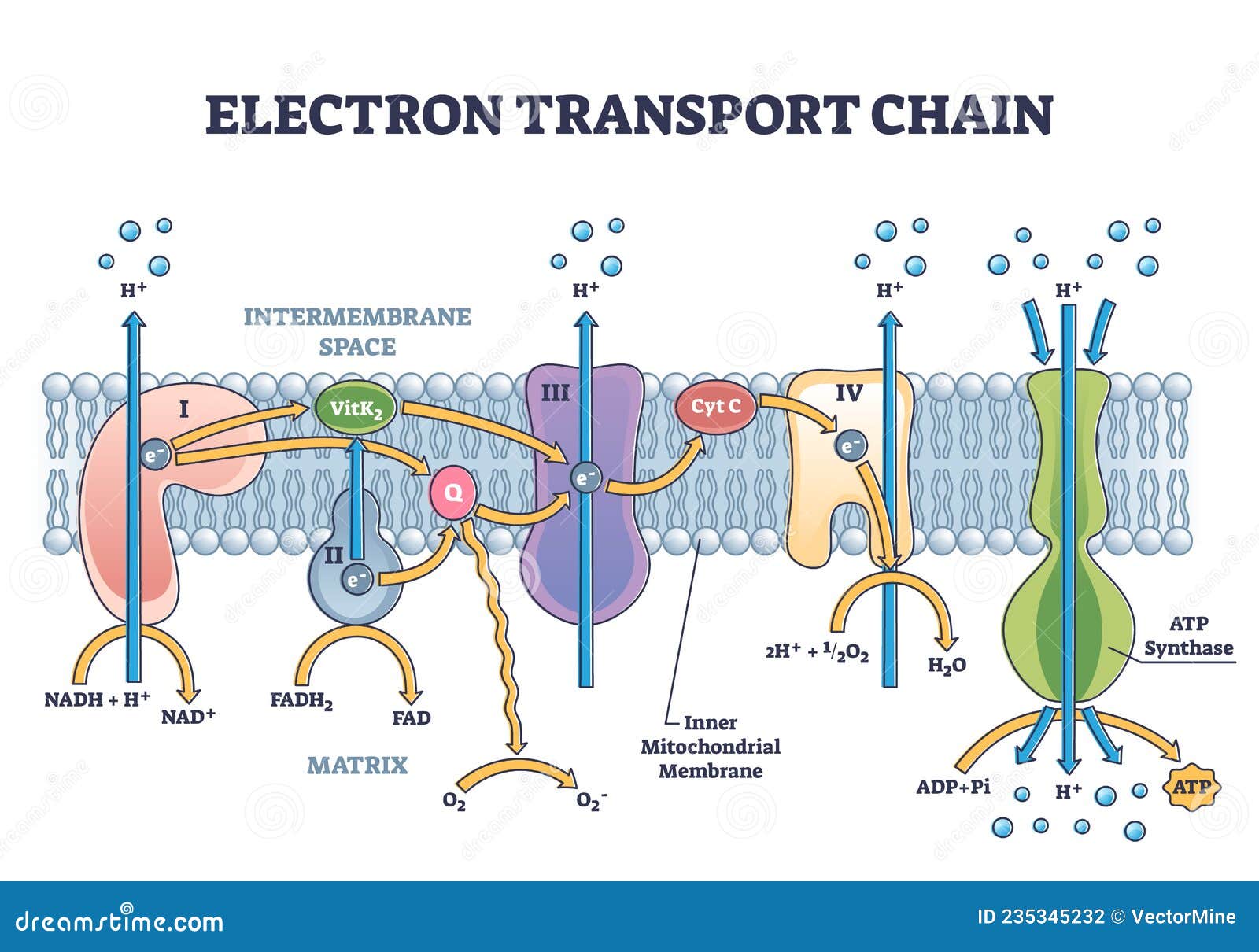
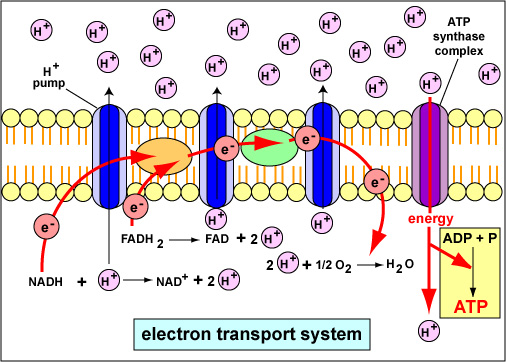
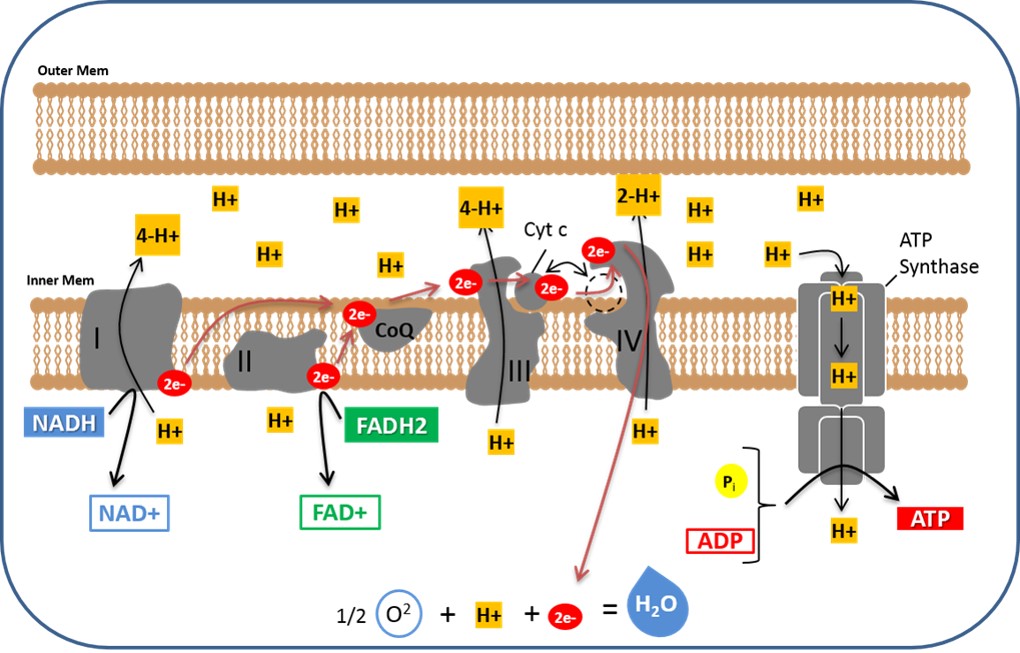
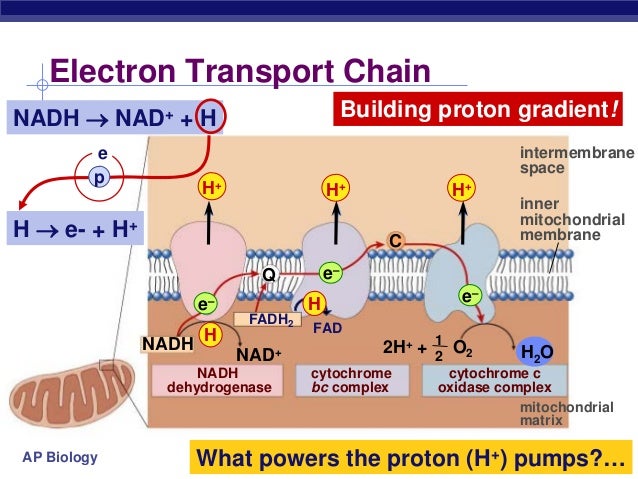
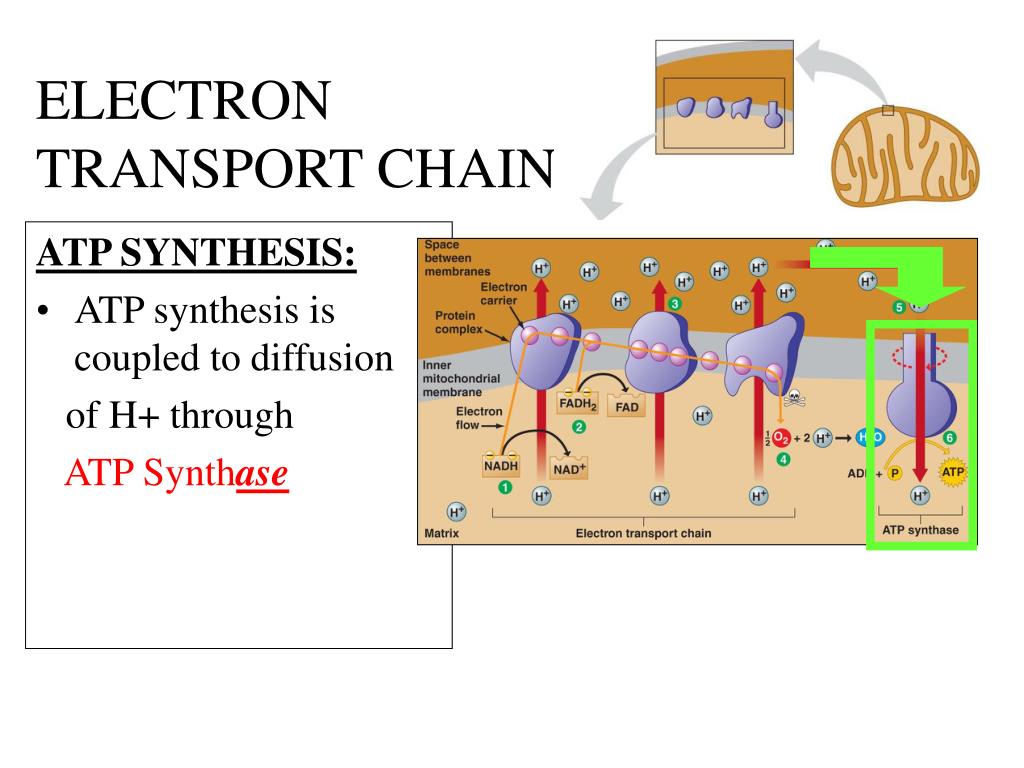
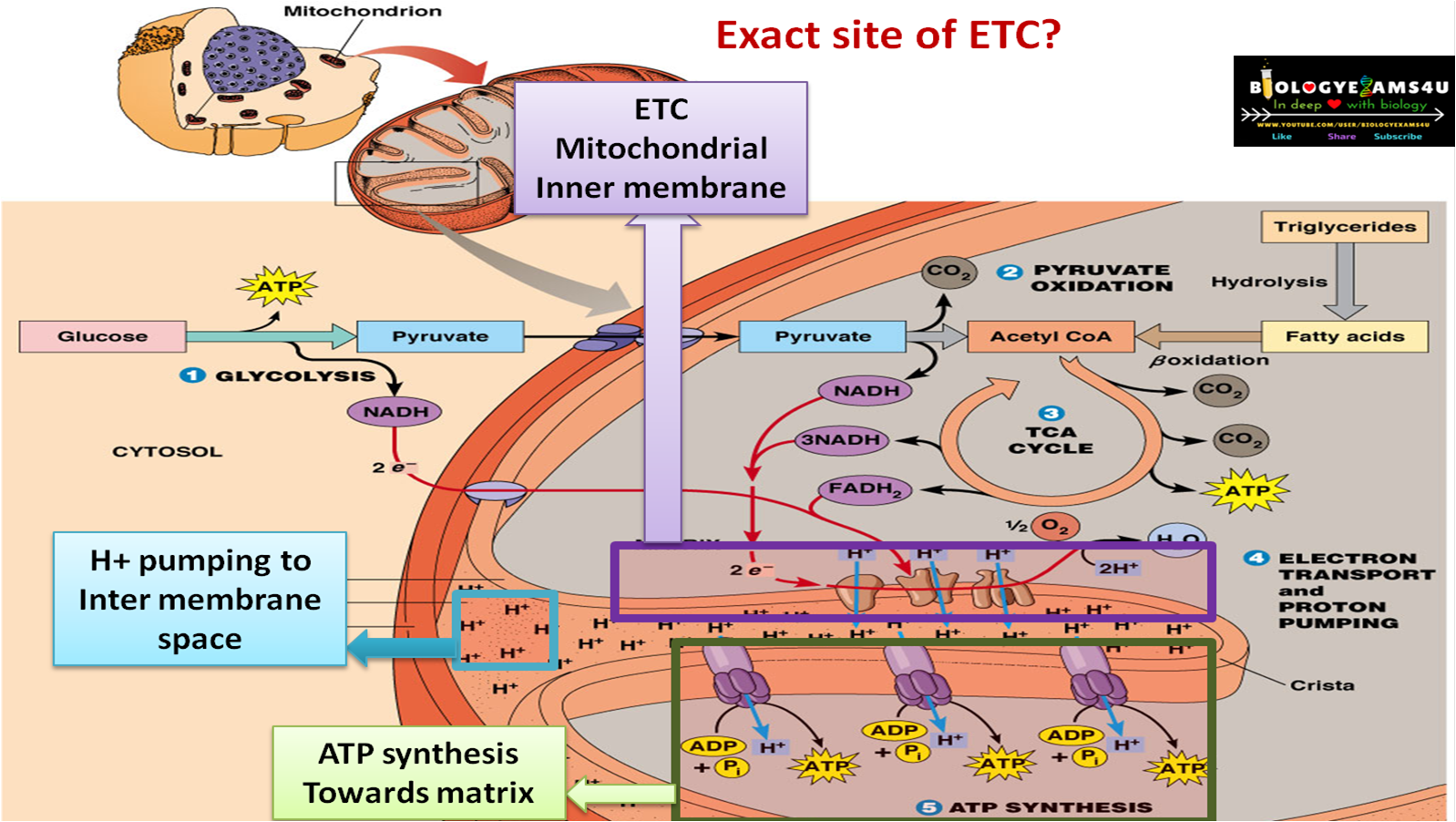
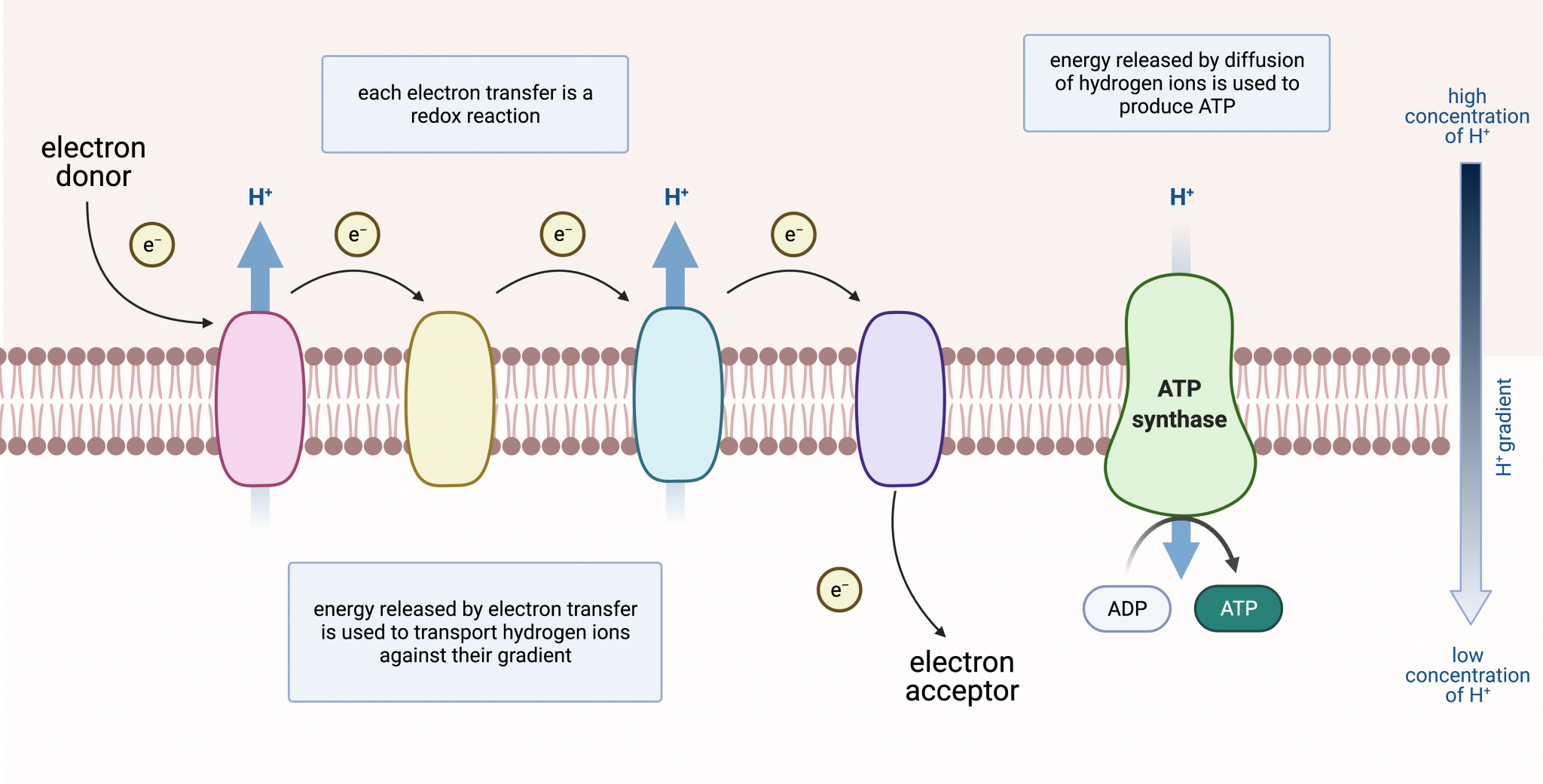
The ETC resides in the inner mitochondrial membrane. It is comprised of several protein complexes (Complex I-IV) and mobile electron carriers (ubiquinone and cytochrome c).
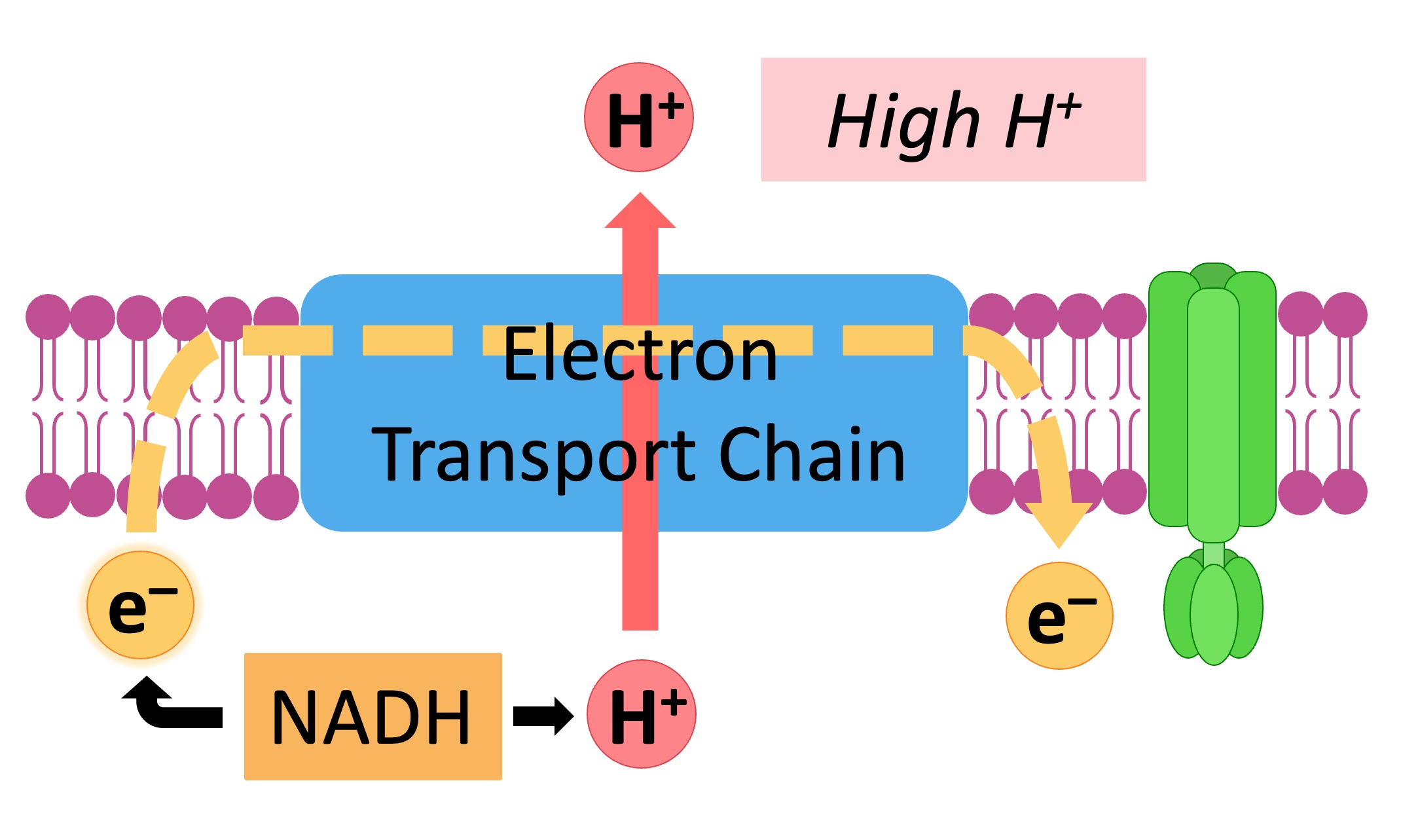
NADH and FADH2 deliver their electrons to these complexes. As electrons move through the chain, protons (H+) are pumped from the mitochondrial matrix into the intermembrane space.
This creates an electrochemical gradient, a reservoir of potential energy. It's like water building up behind a dam, ready to be released.
Chemiosmosis: Harnessing the Gradient
The proton gradient powers ATP synthase. This enzyme acts like a turbine, allowing protons to flow back into the mitochondrial matrix.
This flow drives the rotation of ATP synthase, which catalyzes the reaction that joins ADP and inorganic phosphate to form ATP. This process is called chemiosmosis.
The Elusive Number: How Much ATP?
Estimating the precise ATP yield of the ETC is challenging. The theoretical maximum is often presented, but real-world conditions introduce inefficiencies.
Early estimates suggested that each NADH molecule yields around 3 ATP, and each FADH2 yields around 2 ATP. These numbers were based on the P/O ratio (the ratio of ATP molecules produced per atom of oxygen consumed).
However, these ratios are not fixed and can vary. It depends on factors such as the efficiency of the proton pumps and the "leakiness" of the inner mitochondrial membrane. Leakiness refers to protons flowing back into the matrix without going through ATP synthase.
A More Realistic Range
More recent research suggests a more realistic range. For each NADH, approximately 2.5 ATP molecules are produced.
Each FADH2 generates about 1.5 ATP molecules. This reflects the energy cost of transporting NADH produced in the cytoplasm into the mitochondria.
These adjustments account for proton leakage and other energy-consuming processes. This make them a more accurate reflection of cellular realities.
Accounting for the Entire Process
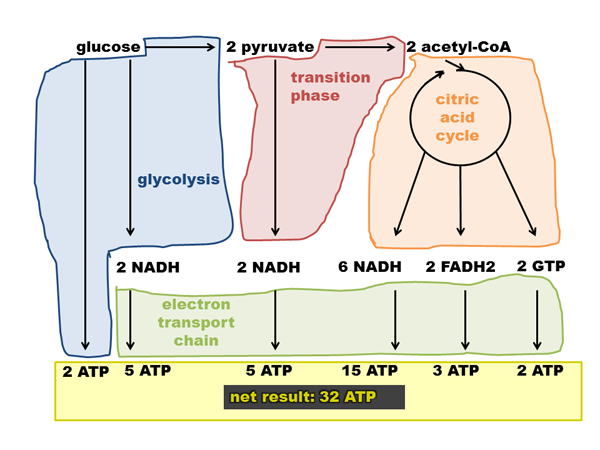
To calculate the total ATP yield, we must consider all stages of cellular respiration. Glycolysis produces 2 ATP and 2 NADH.
The conversion of pyruvate to acetyl-CoA generates 2 NADH. The Krebs cycle yields 2 ATP, 6 NADH, and 2 FADH2.
Summing up the ATP produced directly and through the ETC (using the updated NADH and FADH2 values), the yield is closer to 30-32 ATP per glucose molecule.
Factors Affecting ATP Production
Many factors influence the actual ATP yield. These include the cell type, metabolic state, and the availability of oxygen.
The efficiency of the ETC can also be affected by certain toxins and drugs. For example, cyanide blocks the ETC, preventing ATP production.
Uncoupling proteins can also affect ATP production. These proteins create proton leaks, dissipating the proton gradient as heat rather than generating ATP. This process, called non-shivering thermogenesis, is important for maintaining body temperature in hibernating animals and infants.
The Significance of ATP
ATP is the primary energy currency of the cell. It powers virtually all cellular processes, from muscle contraction to protein synthesis.
A sufficient supply of ATP is crucial for maintaining cell function and survival. Disruptions in ATP production can lead to various diseases.
Understanding the intricacies of ATP production is vital for understanding many biological processes. This has implications for the treatment of metabolic disorders and other diseases.
Conclusion
While the textbook figure of 36-38 ATP per glucose molecule is a useful starting point, a more realistic estimate is around 30-32. This reflects the inherent inefficiencies of the process and the complexities of cellular metabolism.
The electron transport chain remains a marvel of biological engineering. Its efficiency, though not perfect, sustains life as we know it.
As scientists continue to delve deeper into the intricacies of cellular respiration, we can expect our understanding of ATP production to continue to evolve. It's a microscopic dance, crucial to life, and it's always worth watching.

:max_bytes(150000):strip_icc()/ATP_ADP-358b5b4c26b443629b0f3ab9044bfbb1.jpg)