How Much Does An Amino Acid Weigh
Imagine a single grain of sand, almost invisible to the naked eye, yet containing countless atoms within its tiny structure. Now, picture something far smaller – an amino acid, one of the building blocks of life, a fundamental component in constructing the vast and intricate machinery of our bodies. These minuscule molecules are so integral to our existence, yet their individual weight is almost incomprehensible.
This article delves into the surprisingly complex question of how much an amino acid weighs. We'll explore the factors that influence their mass, the units used to measure them, and why understanding their weight is crucial in fields ranging from biochemistry to nutrition.
The Atomic World of Amino Acids
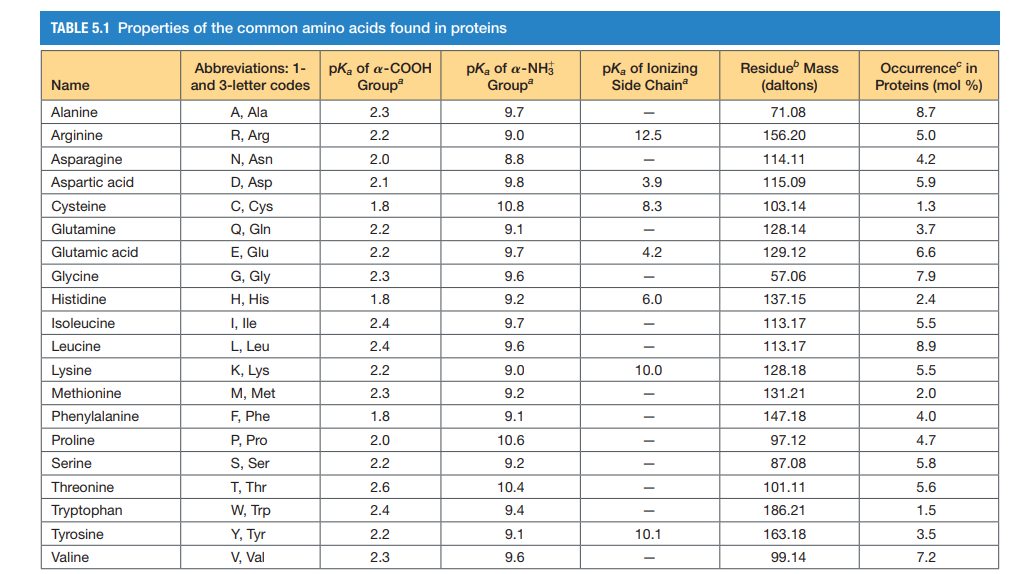
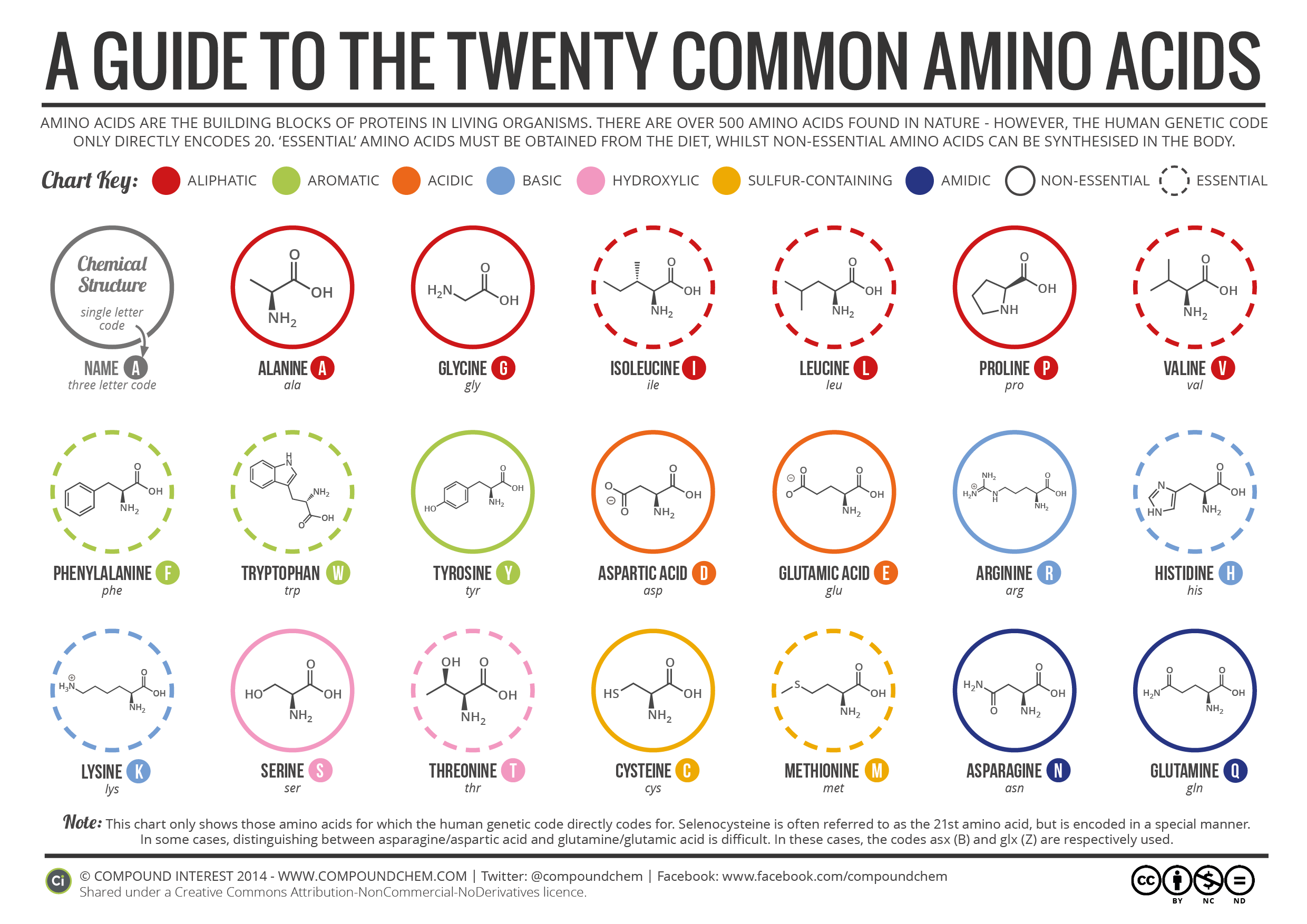
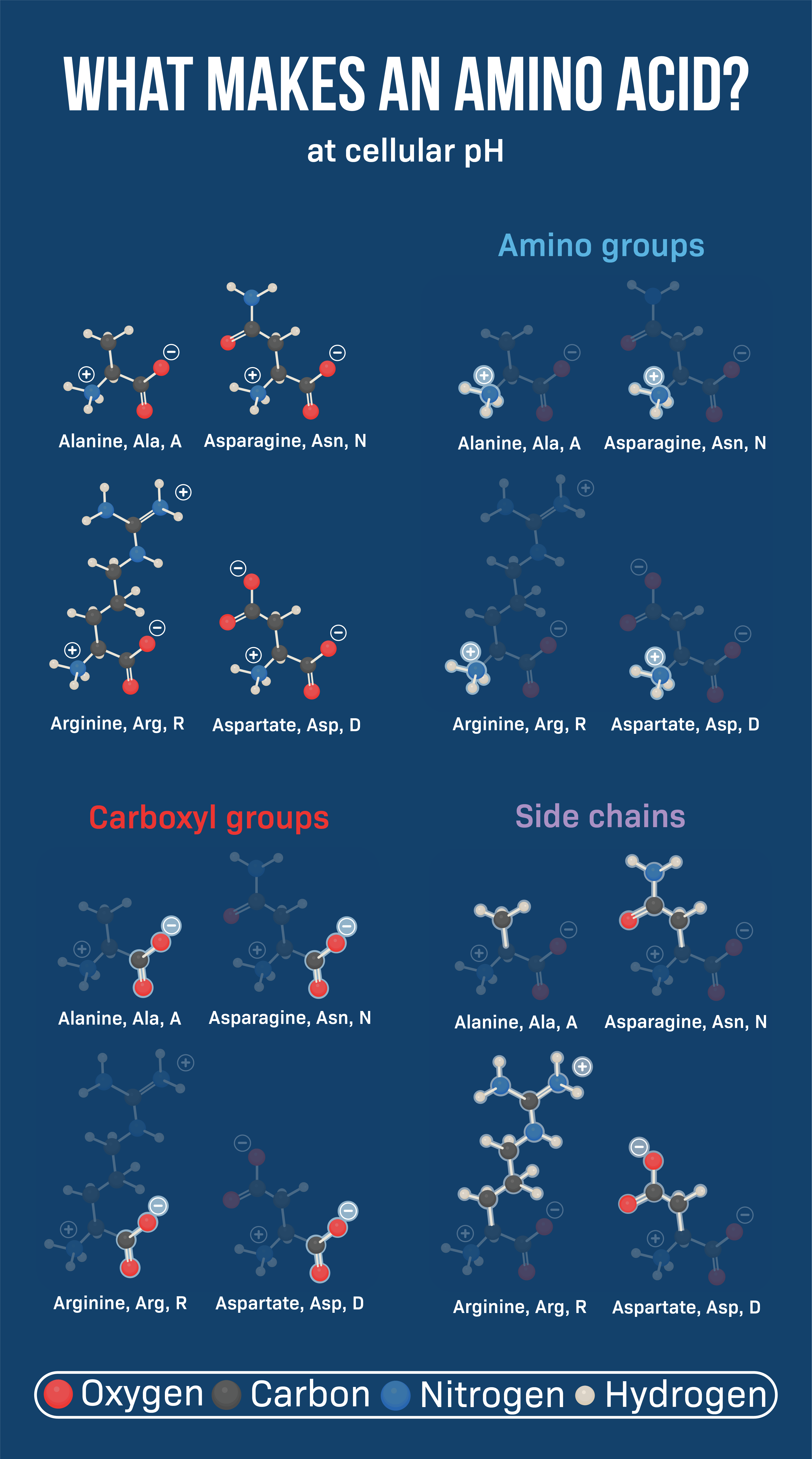
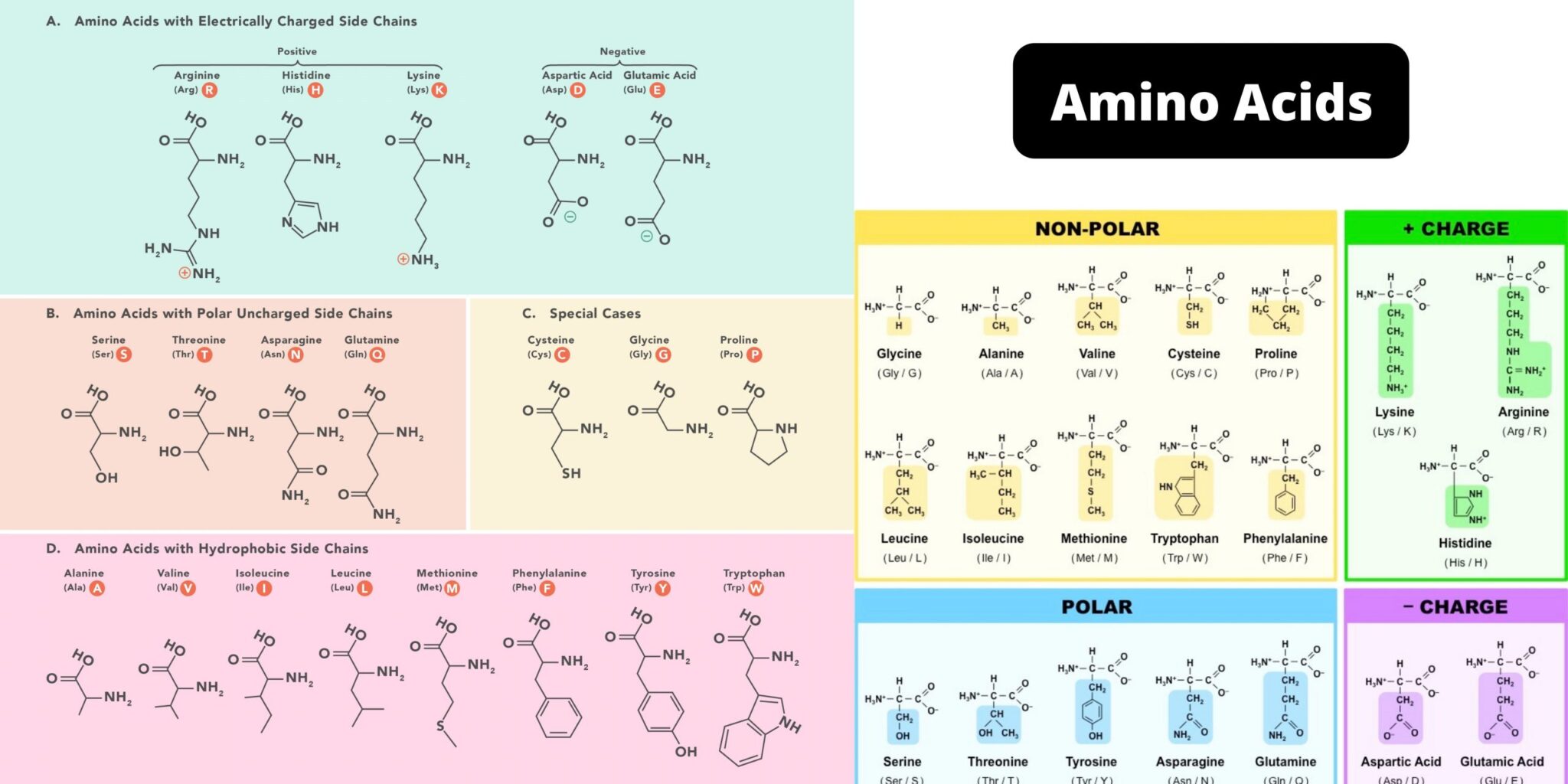
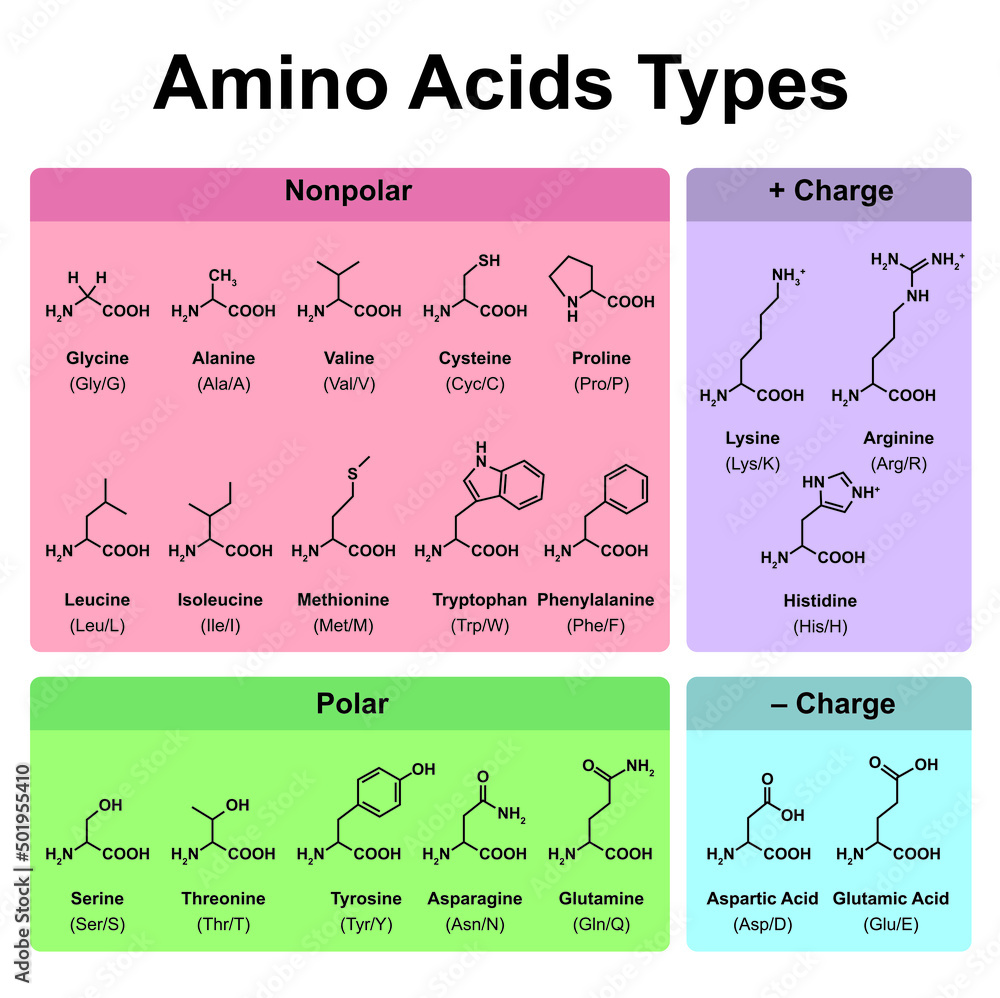
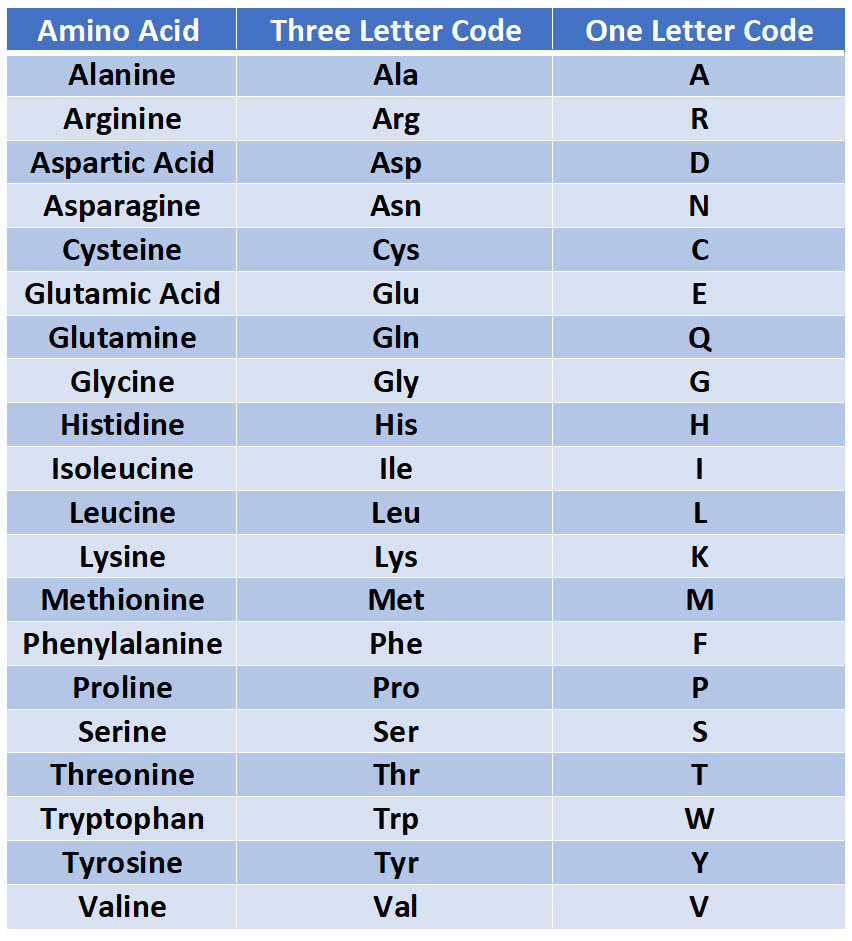
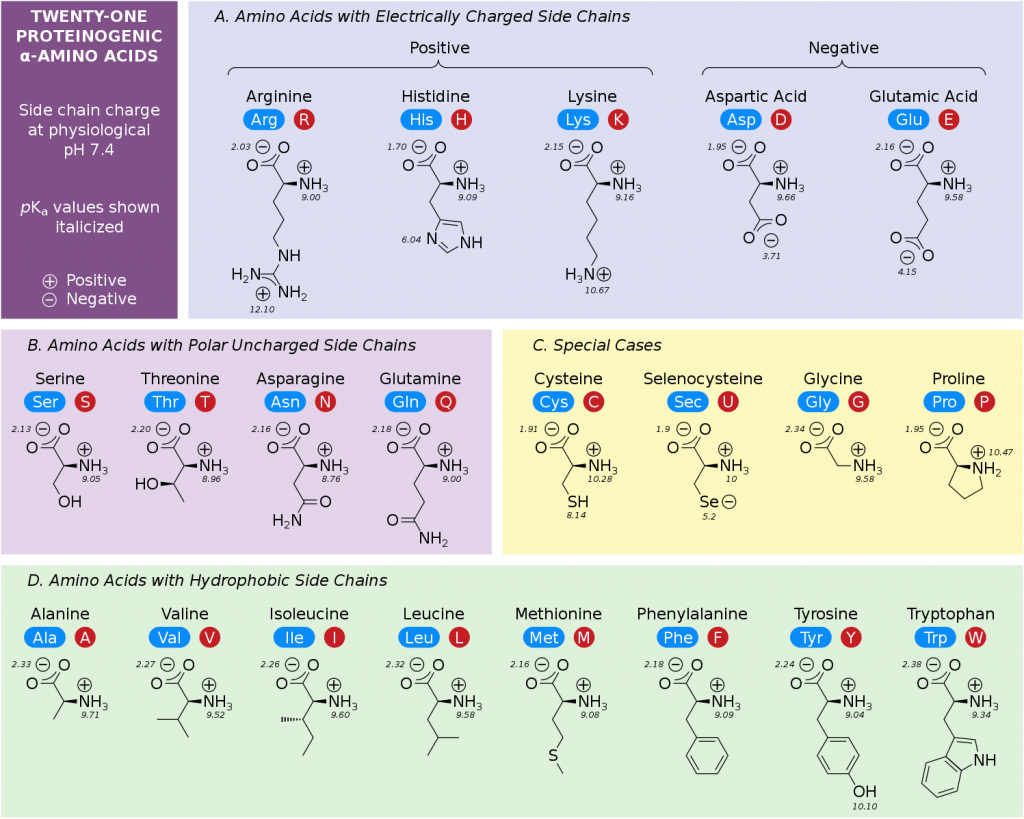
Amino acids are the fundamental units that make up proteins. Each amino acid molecule contains a central carbon atom bound to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a distinctive side chain, also known as an R-group.
This R-group is what differentiates the 20 common amino acids found in proteins. These R-groups vary in size, shape, charge, hydrophobicity, and reactivity, leading to the incredible diversity of protein structures and functions.
Measuring the Immeasurable: Atomic Mass Units
The weight of an amino acid is far too small to be measured in grams or kilograms. Instead, scientists use atomic mass units (amu) or Daltons (Da). One atomic mass unit is defined as 1/12 of the mass of a carbon-12 atom.
This provides a manageable scale for discussing the masses of atoms and molecules. For comparison, a single hydrogen atom weighs approximately 1 amu.
Calculating Amino Acid Weight: It's All About the Atoms
The weight of an amino acid is determined by the sum of the atomic weights of all the atoms that make up the molecule. This includes the carbon, hydrogen, nitrogen, oxygen, and any other atoms present in the R-group.
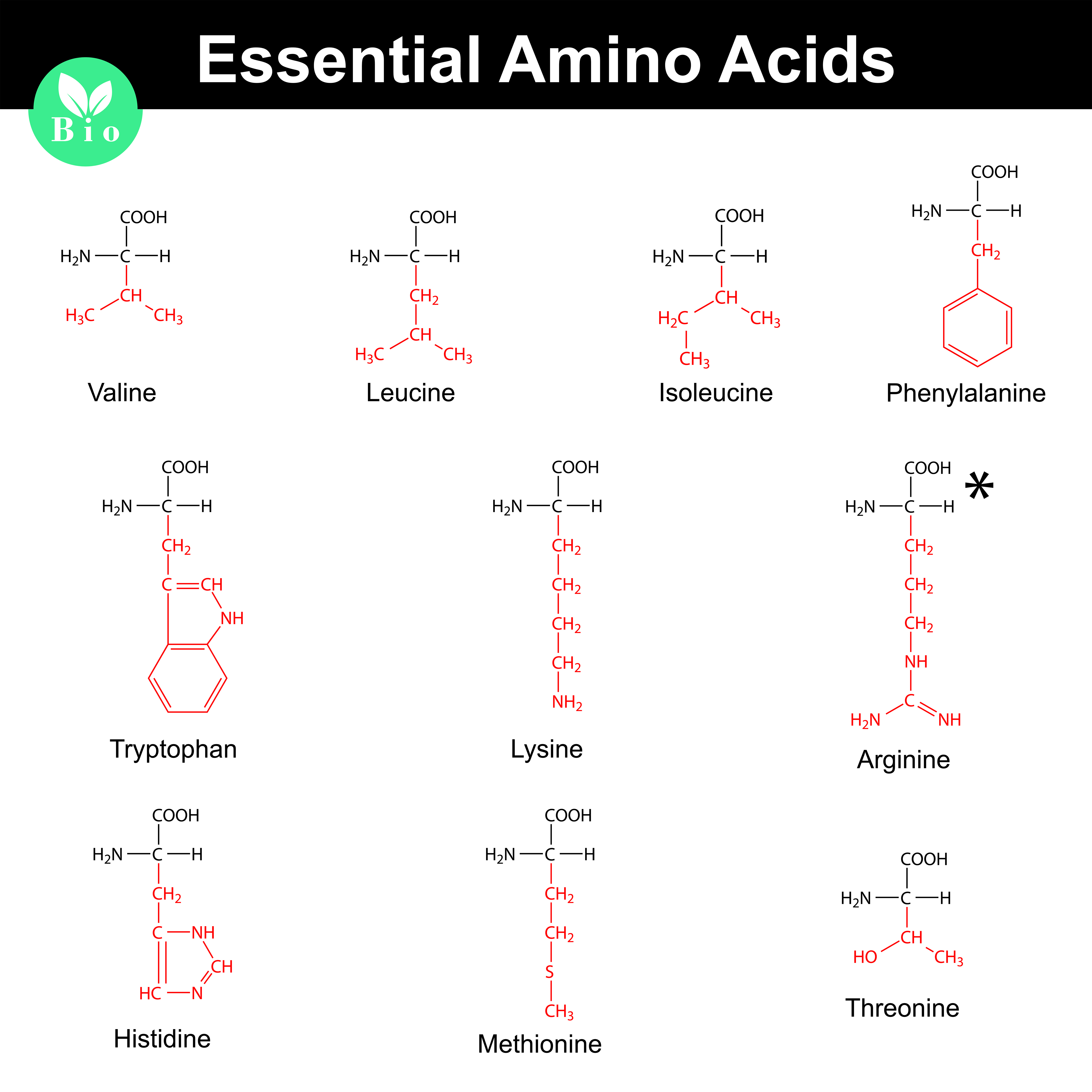
Since each amino acid has a unique R-group, their weights vary. Glycine, the simplest amino acid, has the smallest R-group (just a hydrogen atom) and is therefore the lightest.
Tryptophan, with its large and complex indole ring in its R-group, is one of the heaviest. The average molecular weight of an amino acid is often cited as around 110 Da, but this is just an average.
Individual amino acids can range from roughly 75 Da (Glycine) to around 204 Da (Tryptophan). This range highlights the significant differences in mass due to the varying composition of the R-groups.
The Importance of Knowing Amino Acid Weight
Understanding the weight of amino acids is crucial in various scientific fields. In biochemistry, it's essential for calculating the molecular weight of proteins.
This is important for techniques like mass spectrometry, which is used to identify and quantify proteins based on their mass-to-charge ratio. Knowing the weight of individual amino acids helps researchers decipher complex protein structures.
In nutrition, knowledge of amino acid weights helps in calculating the nutritional value of foods. Scientists use this information to determine the protein content and quality of different food sources.
Understanding the amino acid composition of food is vital for formulating balanced diets and addressing nutritional deficiencies. This becomes especially important for individuals with specific dietary needs or restrictions.
Beyond the Individual: Amino Acids in Chains
While understanding the weight of individual amino acids is important, it's also essential to consider how they combine to form proteins. When amino acids join together through peptide bonds, a molecule of water (H2O) is released.
Therefore, the weight of a protein is not simply the sum of the weights of its individual amino acids. The weight of the water molecules removed during peptide bond formation must be subtracted to obtain the accurate molecular weight of the protein.
This is a crucial consideration when analyzing protein structures and functions. Ignoring the weight of the removed water molecules can lead to significant errors in calculations.
A Tiny Weight, A Mighty Impact
The weight of an amino acid, though incredibly small, plays a significant role in the structure, function, and behavior of proteins. It influences how proteins fold, interact with other molecules, and carry out their diverse biological functions.
The precise mass of each amino acid contributes to the overall architecture of these essential biomolecules. This makes even slight variations in weight potentially impactful on biological processes.
For instance, differences in amino acid weight can affect the rate of protein synthesis or the stability of protein structures. These seemingly minor differences can have significant consequences for cellular function and overall health.
Technological Advances in Weight Measurement
The accurate measurement of amino acid and protein weights has been greatly enhanced by technological advancements. Techniques like mass spectrometry have revolutionized the field of proteomics, allowing scientists to identify and quantify proteins with unprecedented precision.
Mass spectrometry separates molecules based on their mass-to-charge ratio, providing a detailed fingerprint of the protein composition. These technologies are constantly improving, enabling scientists to delve deeper into the complexities of the proteome.
The ability to accurately measure the weight of amino acids and proteins is crucial for advancing our understanding of biology and developing new diagnostic and therapeutic tools.
The Broader Perspective: A World Built on Small Things
Thinking about the weight of an amino acid offers a humbling perspective on the intricate world of molecular biology. It reminds us that even the smallest components can have enormous significance in the grand scheme of life.
These seemingly insignificant molecules are the building blocks upon which all life is built. They underpin the complex processes that sustain us.
Understanding the weight of these fundamental components provides a powerful reminder of the precision and elegance of nature's design. It underscores the importance of continued research and exploration in the field of biochemistry.
The quest to understand the weight of an amino acid, seemingly simple, unlocks a deeper understanding of the very fabric of life itself. It highlights the profound impact of even the smallest elements in the intricate tapestry of existence. It underscores the importance of continued exploration in the microscopic world, where unseen forces shape our macroscopic reality.