How Much Mitragynine In A Gram
The question of how much mitragynine is present in a gram of kratom is far from a simple one, carrying significant implications for users, researchers, and regulators alike. Variances in mitragynine content affect the herb's effects and potentially its risks, making standardization and accurate labeling crucial for informed decisions.
This article dives deep into the complexities of mitragynine concentrations in kratom, exploring the factors that influence these levels, the methods used to measure them, and the ongoing debates surrounding quality control and dosage recommendations. Understanding these nuances is essential for navigating the evolving landscape of kratom use and regulation.
Understanding Mitragynine and Kratom
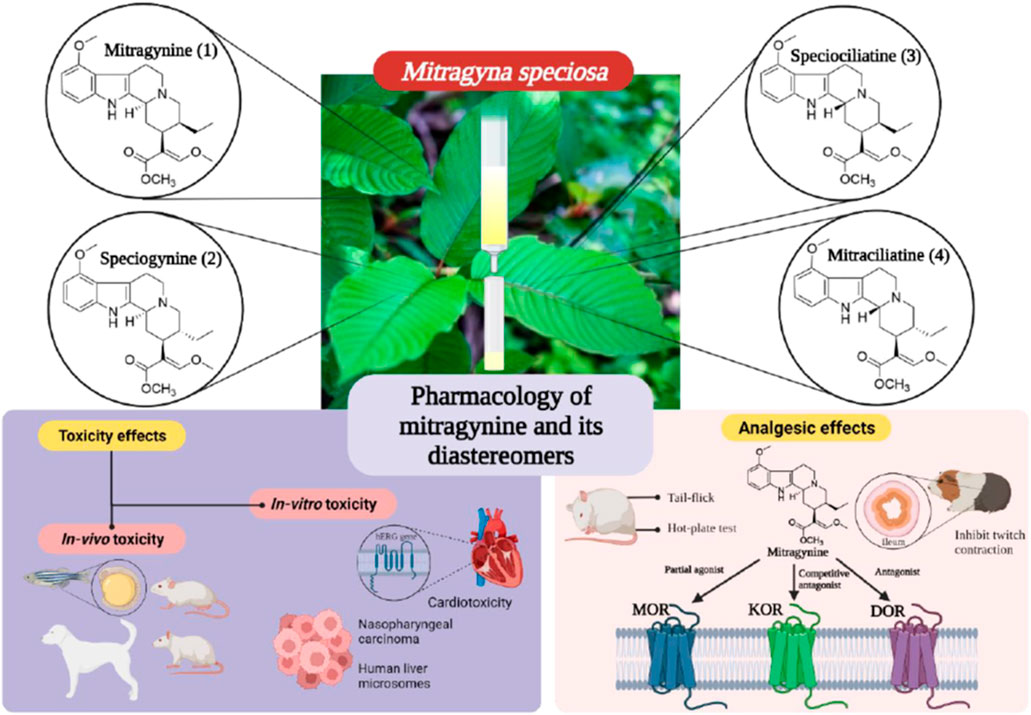
Kratom, derived from the Mitragyna speciosa tree, is a plant indigenous to Southeast Asia. Its leaves contain various alkaloids, with mitragynine being the most prominent and widely studied. This alkaloid interacts with opioid receptors in the brain, leading to stimulant effects at lower doses and analgesic effects at higher doses.
The precise concentration of mitragynine dictates the intensity and type of effects experienced by users, which can range from increased energy and focus to pain relief and relaxation.
The Variability of Mitragynine Content
The mitragynine content in kratom is not consistent; it varies widely due to several factors. These factors include the geographical origin of the plant, the specific strain (e.g., Maeng Da, Bali, Thai), the age of the leaves, and the drying and processing methods used after harvesting.
Soil composition, sunlight exposure, and even seasonal changes can impact the alkaloid profile of the kratom leaves. Consequently, one gram of kratom powder can contain anywhere from less than 0.5% to over 2% mitragynine.
Measuring Mitragynine: Analytical Methods
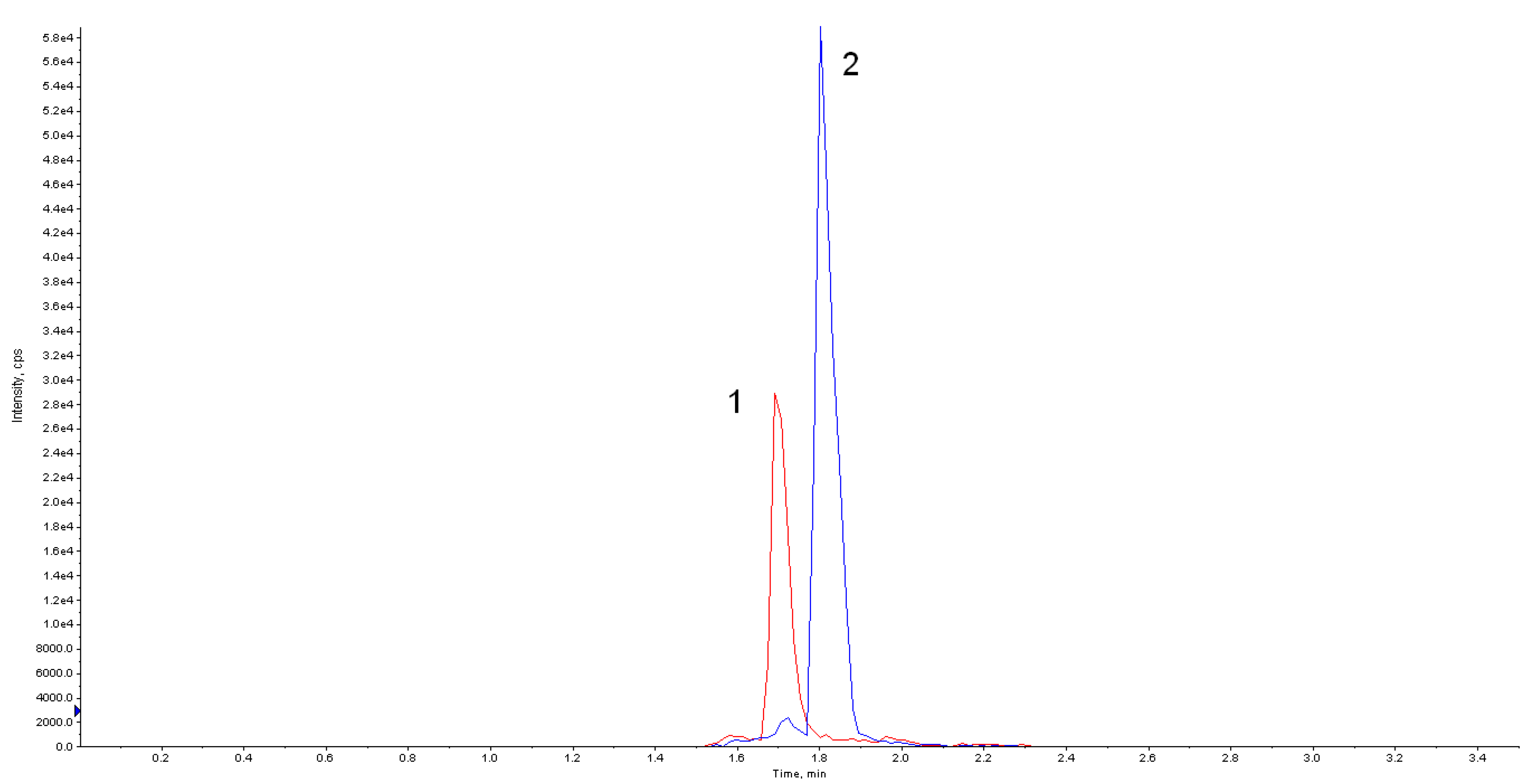
Accurately determining the mitragynine content requires sophisticated analytical techniques. High-Performance Liquid Chromatography (HPLC) is the most common method, offering precise quantification of alkaloids in kratom samples.
Other methods, such as Gas Chromatography-Mass Spectrometry (GC-MS), are also used but HPLC remains the gold standard for its accuracy and reliability. These methods involve extracting alkaloids from the kratom sample and then separating and measuring them using chromatography.
Research Findings on Mitragynine Concentrations
Several studies have investigated the range of mitragynine concentrations in commercially available kratom products. A 2016 study published in the Journal of the American Herbal Products Association analyzed various kratom products and found mitragynine levels ranging from 0.5% to 1.5%.
Other research has indicated even wider ranges, with some samples testing below 0.5% and others exceeding 2%. These disparities highlight the need for better quality control and standardized testing procedures.
The Implications of Variable Mitragynine Levels
The variable mitragynine content presents several challenges. For consumers, inconsistent potency makes it difficult to accurately dose, leading to unpredictable effects and potential adverse reactions.
For researchers, it complicates clinical studies aimed at understanding kratom's therapeutic potential. Furthermore, it hinders regulatory efforts to establish safe and effective guidelines for kratom use.
The Need for Standardization and Quality Control
To address these challenges, there's a growing call for standardization within the kratom industry. This includes establishing minimum and maximum mitragynine content standards for kratom products, as well as implementing rigorous testing protocols.
Organizations like the American Kratom Association (AKA) are advocating for the adoption of Good Manufacturing Practices (GMP) and third-party lab testing to ensure product quality and safety. The AKA's Kratom Consumer Protection Act, proposed in several states, includes provisions for kratom testing and labeling.
Consumer Awareness and Responsible Use
Until comprehensive regulations are in place, consumers must exercise caution and seek out reputable vendors who provide transparent information about their products. This includes looking for certificates of analysis (COAs) from third-party labs that verify the mitragynine content and purity of the kratom.
Starting with low doses and gradually increasing them while monitoring the effects is crucial. Consumers should also be aware of the potential risks associated with kratom use, including dependence and withdrawal symptoms.
The Regulatory Landscape
The regulatory landscape surrounding kratom is complex and varies significantly across different regions. Some countries and states have banned kratom, while others have implemented regulations regarding its sale and use.
The U.S. Food and Drug Administration (FDA) has issued warnings about the safety of kratom and has not approved it for any medical use. However, there is ongoing debate about whether kratom should be regulated as a dietary supplement or a controlled substance.
Looking Ahead: The Future of Mitragynine Research
Continued research is crucial to fully understand the pharmacology of mitragynine and its potential therapeutic applications. Future studies should focus on the long-term effects of kratom use, as well as the optimal dosage for different conditions.
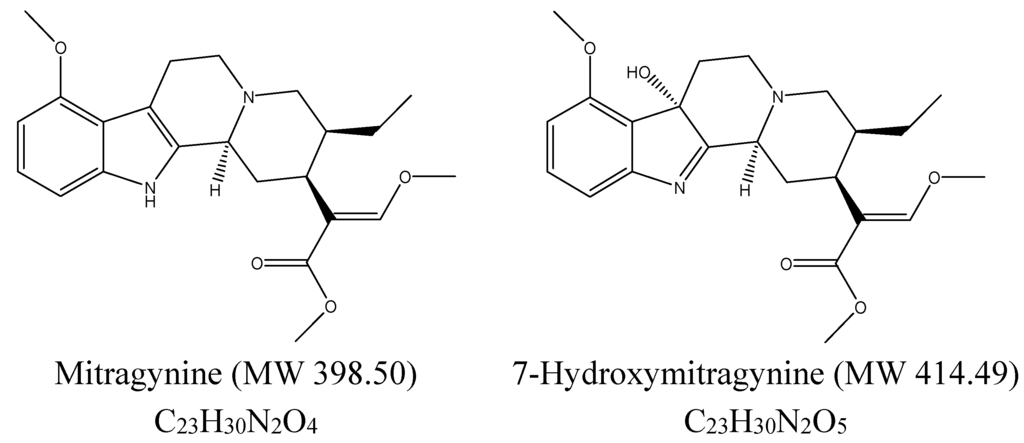
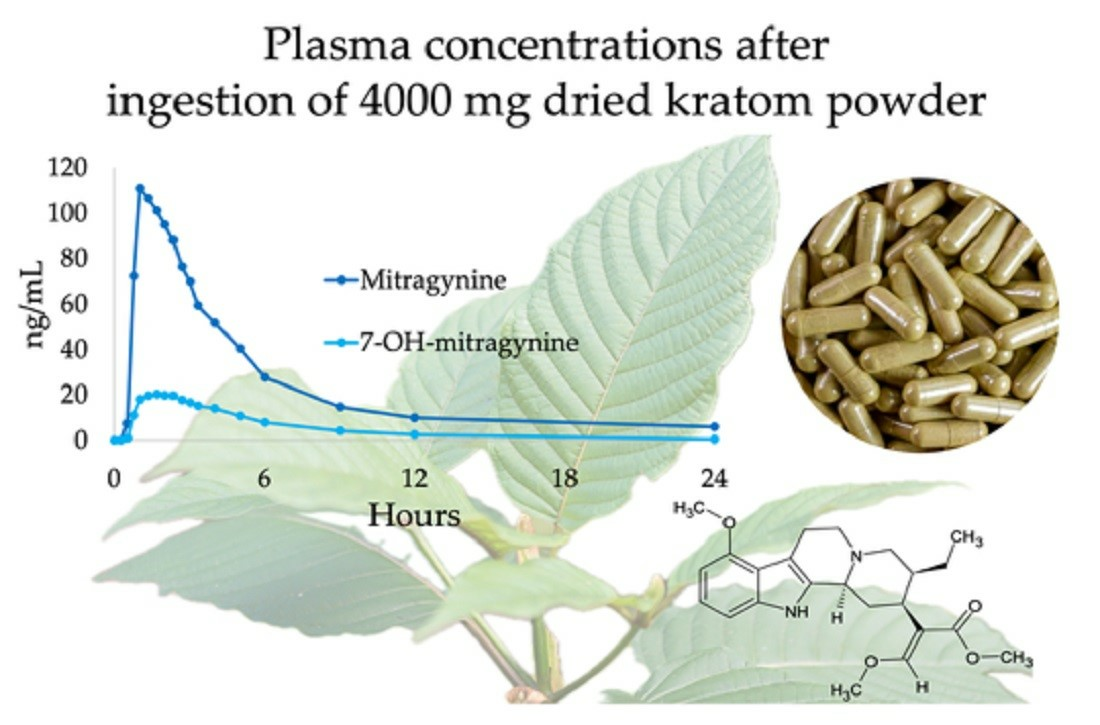
Standardized kratom extracts with known mitragynine concentrations are essential for conducting rigorous clinical trials. Furthermore, research into other alkaloids present in kratom, such as 7-hydroxymitragynine, is needed to gain a more complete picture of the plant's effects.
In conclusion, the amount of mitragynine in a gram of kratom is a critical factor influencing its effects and safety. Addressing the variability in mitragynine content through standardization, quality control, and informed consumer choices is essential for harnessing the potential benefits of kratom while minimizing its risks.