How To Calculate Oxidation No Of Coordination Compound
Chemists worldwide are facing a critical challenge: accurately determining oxidation numbers in coordination compounds. Incorrect calculations can lead to flawed experimental results and misinterpretations of chemical behavior. This article delivers a concise guide to mastering this essential skill, ensuring accuracy in your chemical calculations.
Mastering oxidation number calculation is paramount for correctly predicting compound reactivity, understanding electronic structures, and designing novel materials.
Understanding Oxidation Numbers
Oxidation number, also known as oxidation state, represents the hypothetical charge an atom would have if all bonds were completely ionic. It's a bookkeeping tool, not necessarily reflecting the true charge, but crucial for balancing equations and predicting reactions. For simple ions, it is easy to determine. However, in coordination compounds, things get trickier.
Key Concept: Assigning oxidation numbers follows specific rules to maintain consistency and avoid ambiguity.
Rules for Assigning Oxidation Numbers
Here are the foundational rules: Rule 1: The oxidation number of an element in its elemental form is always 0. Rule 2: The oxidation number of a monatomic ion is equal to its charge.
Rule 3: The sum of oxidation numbers in a neutral compound is 0. For a polyatomic ion, the sum equals the ion's charge. Rule 4: Fluorine always has an oxidation number of -1 in its compounds.
Rule 5: Oxygen usually has an oxidation number of -2, except in peroxides (like H2O2), where it's -1, and with fluorine, where it's positive. Rule 6: Hydrogen usually has an oxidation number of +1, except when bonded to metals in metal hydrides (like NaH), where it's -1.
Calculating Oxidation Numbers in Coordination Compounds
Coordination compounds consist of a central metal atom or ion bonded to surrounding ligands. Ligands are ions or molecules that donate electron pairs to the central metal.
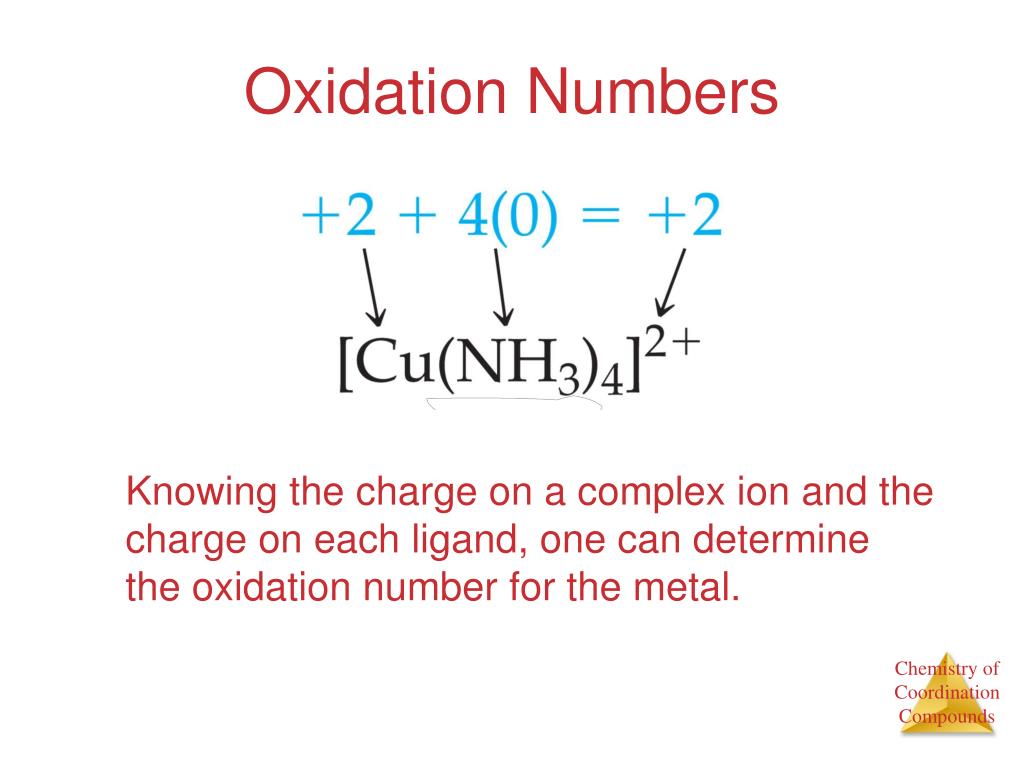
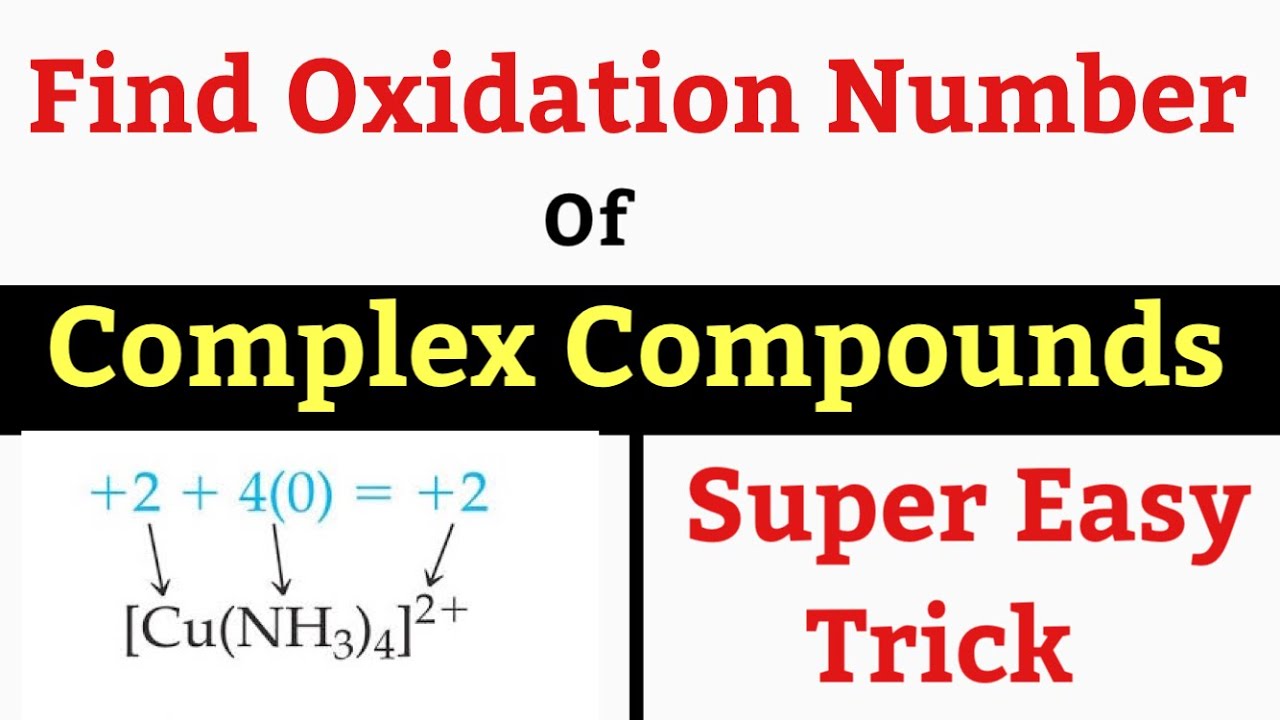
Example: Consider [Co(NH3)5Cl]Cl2. Here, Cobalt (Co) is the central metal, NH3 and Cl are ligands, and Cl2 are counter ions.
Step-by-Step Guide
Step 1: Identify the complex ion and counter ions. In [Co(NH3)5Cl]Cl2, the complex ion is [Co(NH3)5Cl]2+ and the counter ions are 2Cl-.
Step 2: Determine the charge of the complex ion. Since there are two Cl- ions, the complex ion has a +2 charge.
Step 3: Assign oxidation numbers to known ligands. NH3 is a neutral ligand (oxidation number 0), and Cl- has an oxidation number of -1.
Step 4: Set up an equation. Let x be the oxidation number of Cobalt (Co). The equation is: x + 5(0) + (-1) = +2.
Step 5: Solve for x. x - 1 = 2, therefore x = +3. Thus, the oxidation number of Cobalt in [Co(NH3)5Cl]Cl2 is +3.
Common Ligands and Their Charges
Knowing common ligands and their charges is crucial. Some common ligands include H2O (0), NH3 (0), CN- (-1), Cl- (-1), and CO (0). Remember to account for the overall charge of the ligand when calculating the oxidation state of the central metal.
Important Note: Pay close attention to the charge of each ligand and the overall charge of the complex ion. Incorrectly assigning these charges will lead to an incorrect oxidation number for the central metal.
Pitfalls and Considerations
Be cautious with ambidentate ligands (ligands that can bind through different atoms). The binding atom influences the overall charge and oxidation state. Also, remember that oxidation numbers are formal charges; the actual charge distribution can be more complex due to covalent character in the metal-ligand bonds.
Consult reliable sources and practice diligently to refine your skills in assigning oxidation numbers.
Next Steps
Regular practice with varied coordination complexes is key to mastering oxidation number calculations. Review online resources, textbooks, and seek guidance from experienced chemists or instructors to reinforce your understanding. Accurate calculation of oxidation numbers is paramount for successful advancement in inorganic chemistry and related fields.



![How To Calculate Oxidation No Of Coordination Compound [Solved] Find coordination number, oxidation no. o | SolutionInn](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2022/11/6380b7c6075e5_0376380b7c5b10ee.jpg)