How To Find The Formula Weight Of A Compound

In chemistry, determining the formula weight of a compound is a foundational skill, essential for understanding chemical reactions, stoichiometry, and the composition of substances. This seemingly simple calculation is the bedrock upon which more complex chemical analyses are built, making it indispensable for students, researchers, and industry professionals alike.
This article provides a comprehensive guide to calculating formula weight, also known as molecular weight, encompassing the necessary steps, potential pitfalls, and its significance in the broader scientific context. Understanding the formula weight allows chemists to accurately measure reactants and products, predict reaction yields, and ultimately, control and manipulate chemical processes with precision.
Understanding Formula Weight: The Foundation of Chemical Calculations
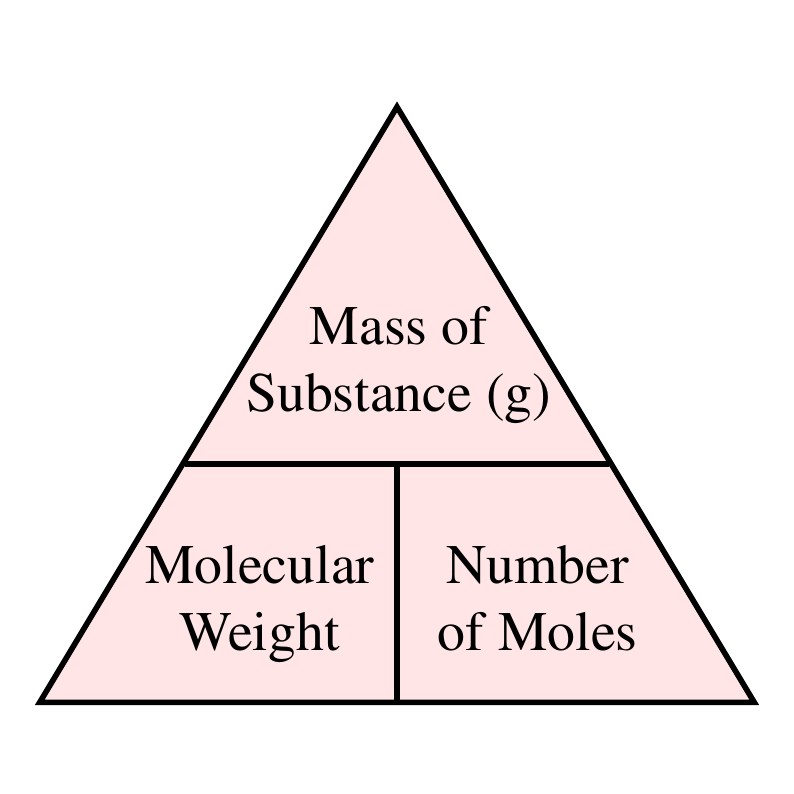
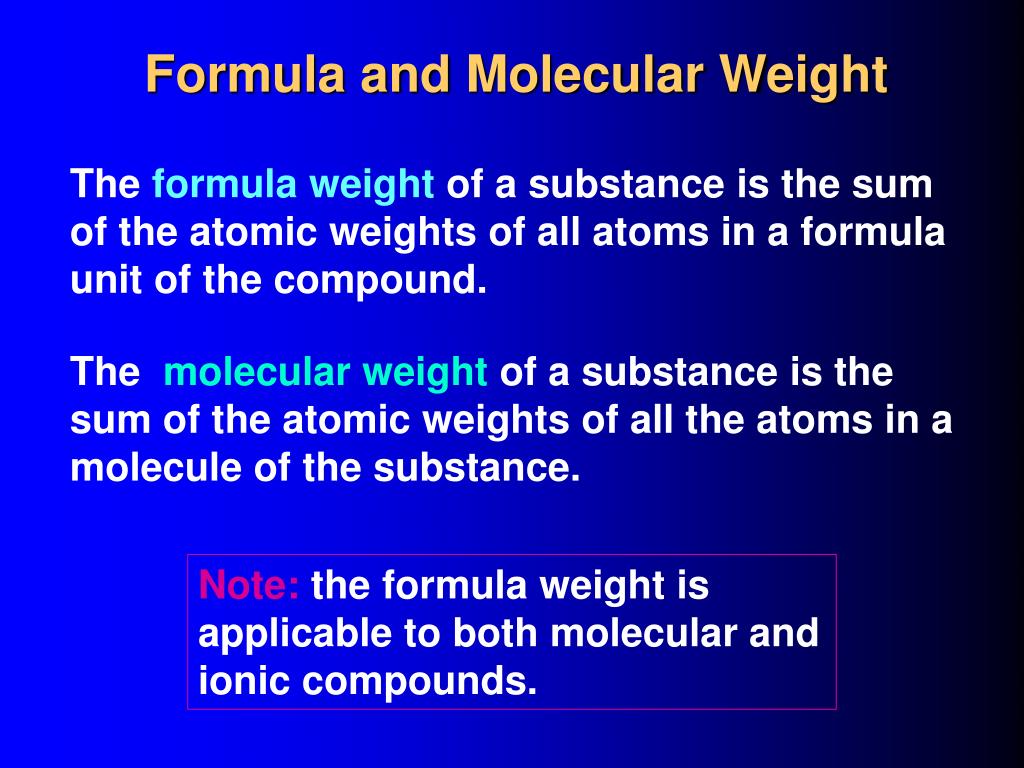
The formula weight, or molecular weight, represents the sum of the atomic weights of all the atoms in a chemical formula. It is expressed in atomic mass units (amu) or, more commonly, in grams per mole (g/mol), which is particularly relevant for laboratory applications.
Accurately determining the formula weight is critical for stoichiometric calculations, converting between mass and moles, and preparing solutions of specific concentrations. Without a solid grasp of this concept, manipulating chemical reactions becomes akin to navigating without a map.
Step-by-Step Guide to Calculating Formula Weight
The process of finding the formula weight involves several key steps, each requiring careful attention to detail.
Step 1: Identify the Chemical Formula. Begin by accurately identifying the chemical formula of the compound in question. For instance, consider water (H2O), sodium chloride (NaCl), or sulfuric acid (H2SO4).
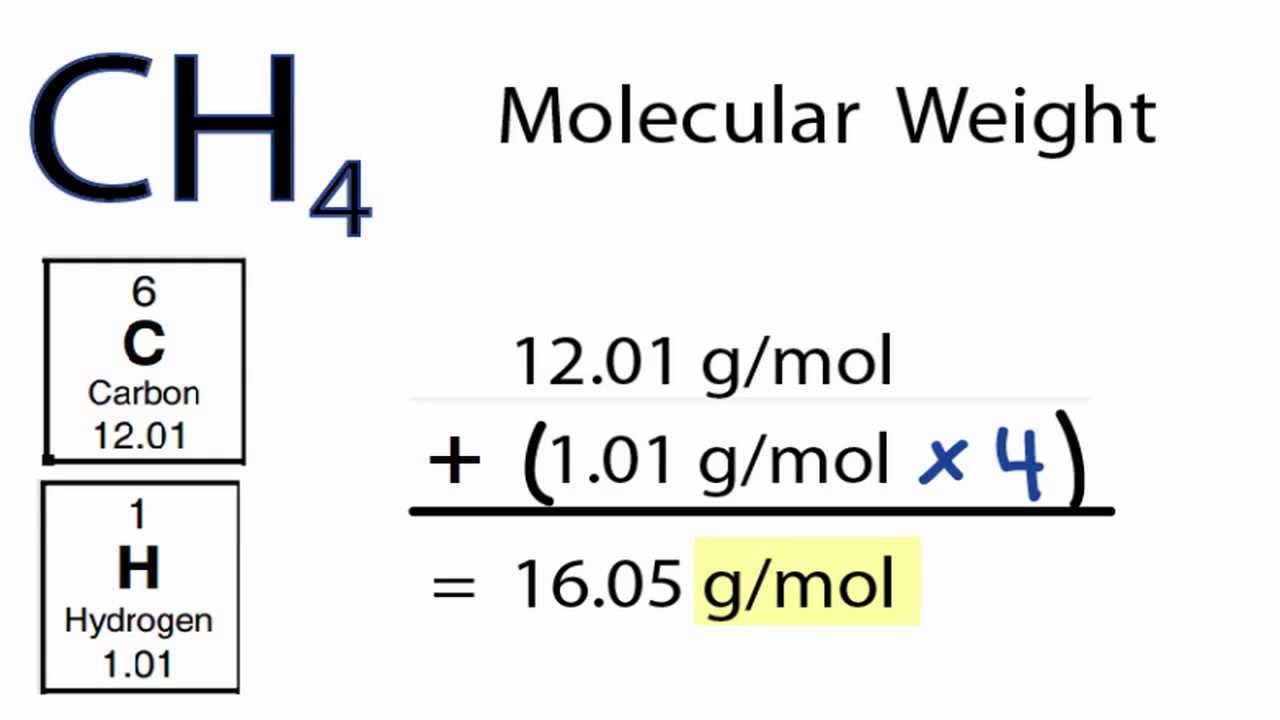
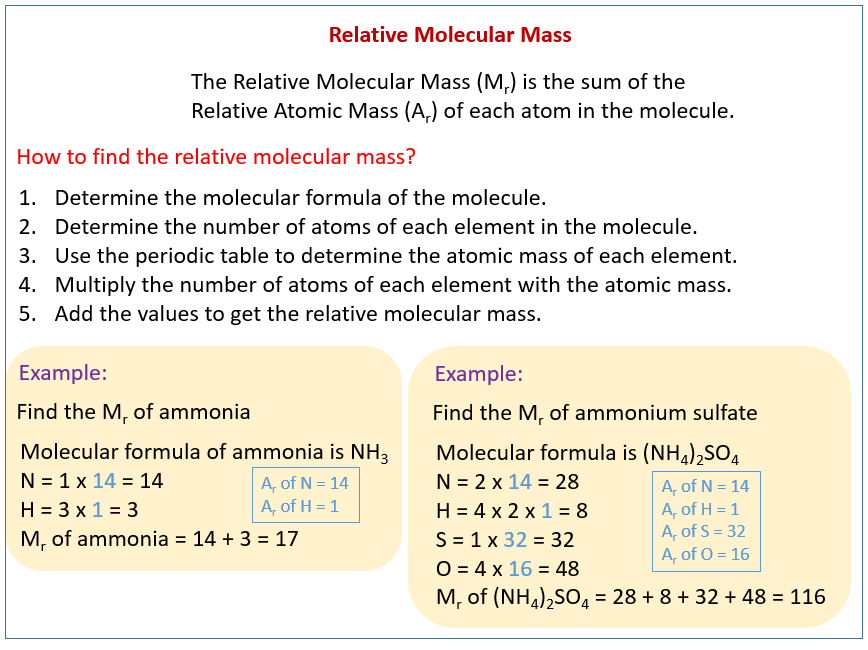
Step 2: Determine the Atomic Weights. Consult a periodic table to find the atomic weight of each element present in the formula. The atomic weight is typically found beneath the element symbol and is often a decimal number.
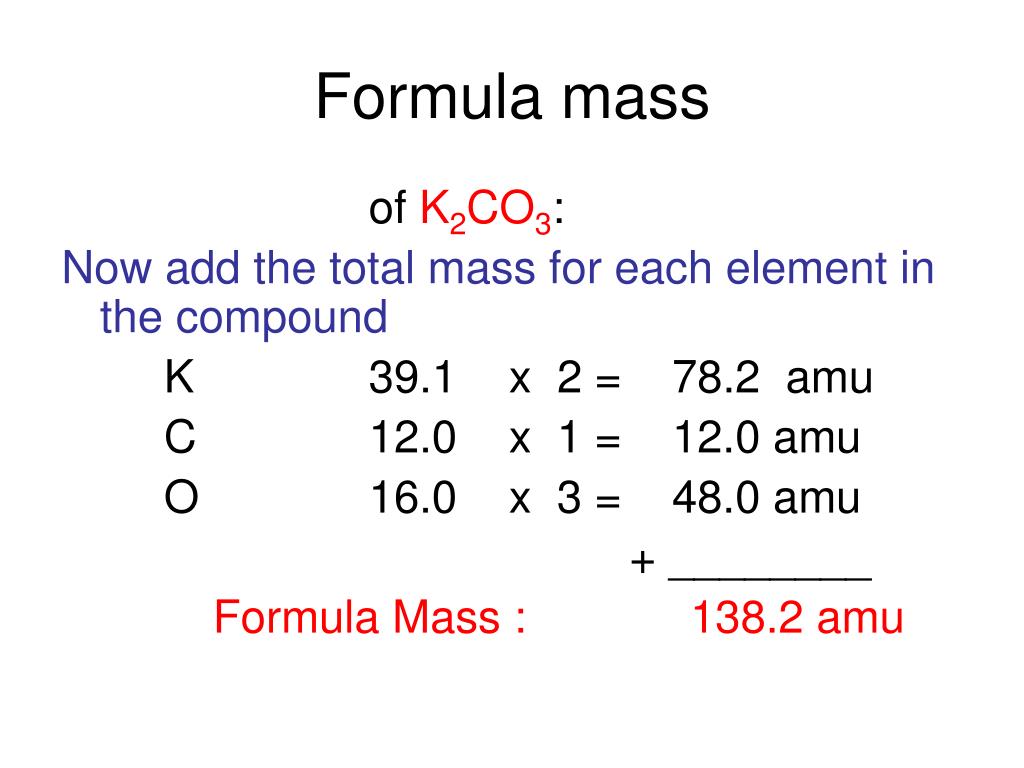
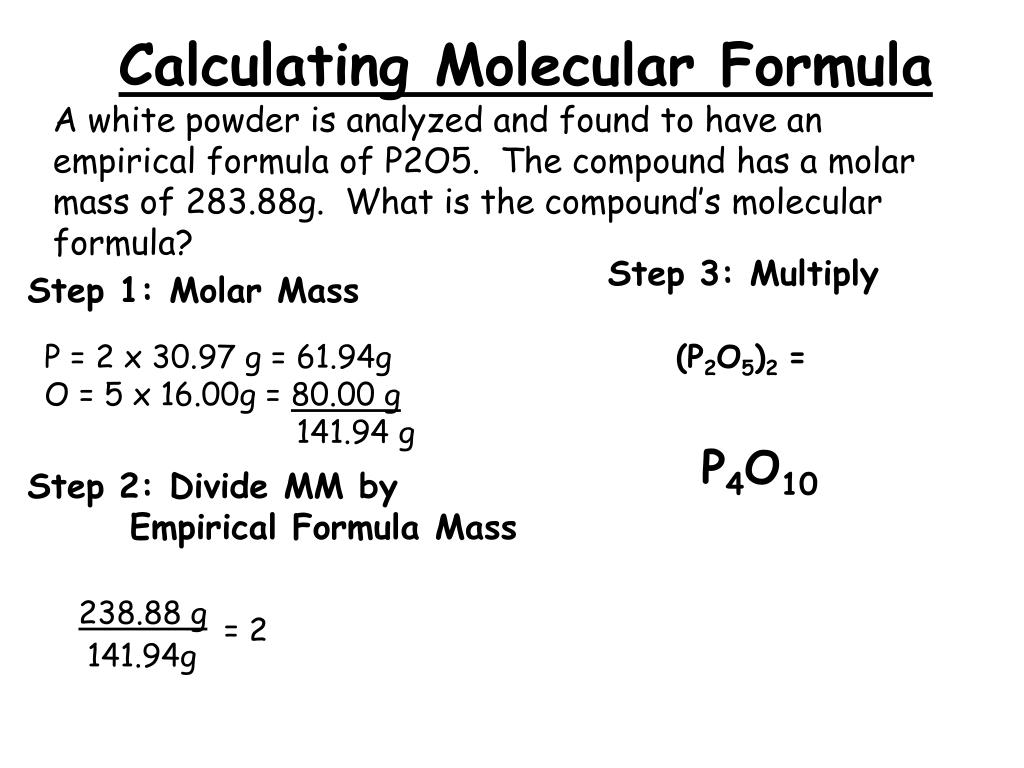
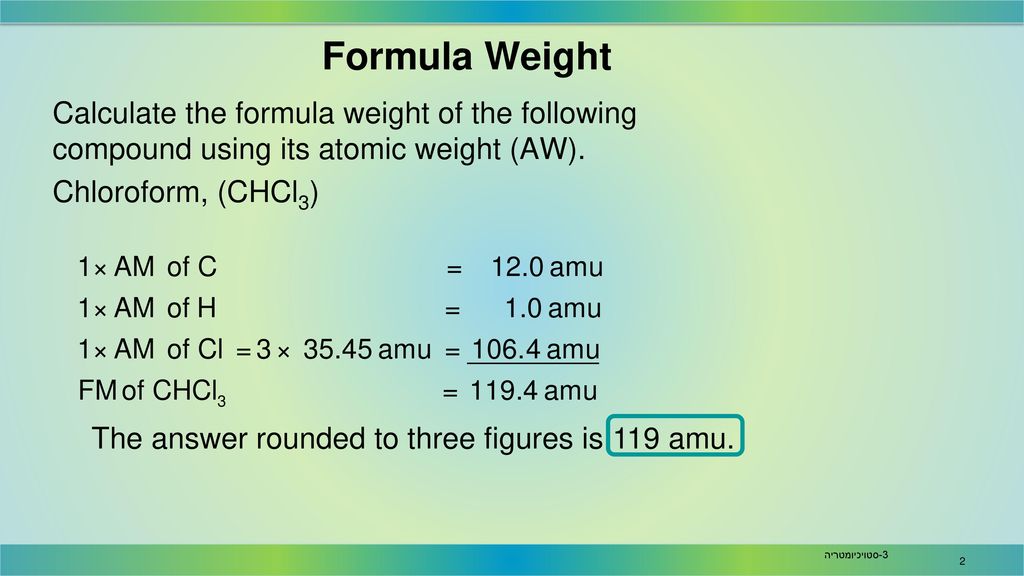
Step 3: Multiply and Sum. Multiply the atomic weight of each element by the number of times it appears in the chemical formula. Then, sum up these values for all the elements to obtain the formula weight.
Example: Calculating the Formula Weight of Water (H2O)
- Atomic weight of Hydrogen (H): ~1.008 amu
- Atomic weight of Oxygen (O): ~16.00 amu
Two hydrogen atoms: 2 * 1.008 amu = 2.016 amu. One oxygen atom: 1 * 16.00 amu = 16.00 amu. Formula weight of H2O: 2.016 amu + 16.00 amu = 18.016 amu (or g/mol).
Common Pitfalls and How to Avoid Them
While the calculation itself is relatively straightforward, several common errors can lead to inaccurate results.
Misreading the Chemical Formula. Ensure the chemical formula is transcribed correctly. Even a small error, like omitting a subscript, can drastically change the formula weight. Double-check all subscripts and element symbols.
Using Inaccurate Atomic Weights. Always use the most accurate and up-to-date atomic weights from a reliable periodic table. Atomic weights can vary slightly depending on the source.
Forgetting to Multiply. Remember to multiply the atomic weight of each element by its corresponding subscript in the chemical formula. This is a common source of error, especially in compounds with multiple atoms of the same element.
Formula Weight vs. Molecular Weight vs. Molar Mass
The terms "formula weight," "molecular weight," and "molar mass" are often used interchangeably, but there are subtle distinctions.
Molecular weight technically refers to the mass of a single molecule and is strictly applicable to covalent compounds. Formula weight is a more general term, applicable to both ionic and covalent compounds. Molar mass is the mass of one mole (6.022 x 1023 entities) of a substance and is expressed in grams per mole (g/mol). In practice, all three terms often refer to the same numerical value, just with different units or contexts.
For example, the formula weight of NaCl can be calculated using the atomic weight of Sodium (~22.99 g/mol) and the atomic weight of Chlorine (~35.45 g/mol). Adding the values, we get 58.44 g/mol. Therefore, the molar mass of NaCl is 58.44 g/mol.
Applications of Formula Weight in Chemistry
The ability to calculate formula weight has myriad applications in chemistry, ranging from basic laboratory procedures to advanced research.
Stoichiometry: Formula weight is crucial for stoichiometric calculations, which involve determining the quantitative relationships between reactants and products in a chemical reaction. It enables chemists to convert between mass and moles, ensuring accurate proportions in chemical reactions. Understanding these proportions is key to maximizing reaction yields.
Solution Preparation: Preparing solutions of specific molar concentrations requires precise knowledge of the solute's formula weight. The molarity of a solution is defined as the number of moles of solute per liter of solution. Calculating the necessary mass of solute involves using its formula weight.
Analytical Chemistry: In analytical chemistry, formula weight is used in quantitative analysis techniques to determine the amount of a specific substance in a sample. Techniques like titration and gravimetric analysis rely heavily on accurate formula weight calculations.
The Future of Formula Weight Calculations: Technology and Innovation
While the fundamental principles of calculating formula weight remain unchanged, technology continues to enhance the process.
Online calculators and software programs provide quick and accurate formula weight calculations, reducing the risk of human error. These tools often incorporate large databases of atomic weights and chemical formulas.
Advanced analytical techniques, such as mass spectrometry, can directly determine the molecular weight of complex molecules with high precision. However, even with these advanced tools, a solid understanding of the underlying principles of formula weight calculation remains essential for interpreting the results and validating the data.
As chemistry advances, the significance of accurate formula weight determination will only increase, ensuring precise control and understanding of chemical processes in diverse fields, from drug discovery to materials science. The concept is a critical stepping stone to many areas of chemistry.



+of+each+of+the+following.jpg)


.PNG)