How To Test For Titanium Toxicity
The gleaming allure of titanium, prized for its strength, lightweight nature, and biocompatibility, has made it a ubiquitous material in medical implants, aerospace engineering, and consumer products. Yet, beneath its seemingly inert surface lies a growing concern: the potential for titanium toxicity. While generally considered safe, the long-term effects of titanium exposure, particularly from nanoparticles released from implants or environmental contamination, are increasingly questioned, prompting researchers and clinicians to seek reliable methods for detection and assessment.
This article delves into the complexities of testing for titanium toxicity, providing a comprehensive overview of available diagnostic methods, their limitations, and the ongoing research aimed at improving our understanding of this emerging health concern. It explores the challenges in accurately measuring titanium levels in the body, differentiating between harmless exposure and toxic accumulation, and interpreting the clinical significance of detected titanium.
The Challenge of Detecting Titanium Toxicity
Measuring titanium levels in the body is far from straightforward. Unlike common heavy metals like lead or mercury, titanium is not easily detected in standard blood or urine tests. The challenge stems from its low concentration in biological tissues and the lack of standardized, widely available testing protocols.
Furthermore, distinguishing between background environmental exposure and clinically relevant levels is crucial. We are all exposed to small amounts of titanium through food, water, and air. The key is to determine when exposure translates into accumulation and potential toxicity.
Existing Methods and Their Limitations
Several methods are currently employed, albeit with varying degrees of accuracy and accessibility, for detecting titanium in biological samples. These include:
- Inductively Coupled Plasma Mass Spectrometry (ICP-MS): This highly sensitive technique can measure trace amounts of titanium in blood, urine, and tissue samples. However, its availability is limited to specialized laboratories, and sample preparation can be complex, potentially leading to contamination or inaccurate results.
- Atomic Absorption Spectrometry (AAS): While less sensitive than ICP-MS, AAS is a more widely available technique for measuring titanium levels. Its limitations lie in its lower detection limits, which may not be sufficient to detect low-level titanium exposure.
- X-ray Fluorescence (XRF): XRF is a non-destructive technique that can be used to analyze the elemental composition of materials, including biological tissues. It is particularly useful for detecting titanium in bone and other hard tissues but may be less sensitive for soft tissues.
- Transmission Electron Microscopy (TEM): TEM is a powerful technique for visualizing nanoparticles, including titanium dioxide (TiO2) nanoparticles, in biological samples. It provides information about the size, shape, and distribution of nanoparticles, but it is labor-intensive and requires specialized expertise.
- Histopathological Analysis: Examining tissue biopsies under a microscope can reveal the presence of titanium particles and signs of inflammation or tissue damage. This method provides valuable information about the local effects of titanium exposure.
Each of these methods has its own strengths and weaknesses. No single test is perfect for diagnosing titanium toxicity, and a combination of approaches may be necessary for a comprehensive assessment.
Interpreting Test Results and Assessing Toxicity
Even with accurate measurement of titanium levels, interpreting the results and determining whether toxicity exists remains a challenge. There are currently no established "safe" or "toxic" thresholds for titanium in biological samples.
Clinicians must consider a variety of factors, including the patient's medical history, symptoms, and the specific type of titanium exposure. For instance, a patient with a failed titanium hip implant may have elevated titanium levels in their surrounding tissues, leading to inflammation and pain. Conversely, a healthy individual with low-level titanium exposure through diet may not experience any adverse effects.
Distinguishing between localized and systemic titanium exposure is also crucial. Localized exposure, such as around an implant, may cause localized inflammation and tissue damage. Systemic exposure, resulting from the distribution of titanium nanoparticles throughout the body, may have broader, less well-understood effects.
Emerging Research and Future Directions
Research into titanium toxicity is an active and evolving field. Scientists are actively exploring the mechanisms by which titanium nanoparticles interact with cells and tissues, and the potential long-term health consequences of chronic exposure.
New and improved methods for detecting titanium are also being developed. These include:
- Single-particle ICP-MS (SP-ICP-MS): This technique can measure the size and concentration of individual titanium nanoparticles, providing more detailed information about the characteristics of titanium exposure.
- Advanced Imaging Techniques: Researchers are exploring the use of advanced imaging techniques, such as multiphoton microscopy and Raman spectroscopy, to visualize titanium nanoparticles in living tissues.
- Biomarkers of Titanium Toxicity: Scientists are searching for biomarkers, such as specific proteins or gene expression patterns, that can indicate early signs of titanium toxicity.
Furthermore, researchers are investigating the potential role of genetics in susceptibility to titanium toxicity. Some individuals may be more sensitive to the effects of titanium exposure due to variations in their genes.
The Need for Standardized Testing and Guidelines
One of the key challenges in addressing titanium toxicity is the lack of standardized testing protocols and clinical guidelines. The development of standardized methods for measuring titanium levels in biological samples, along with clear criteria for interpreting test results, is essential.
This would allow clinicians to accurately assess the risks associated with titanium exposure and provide appropriate medical care. It would also facilitate research into the long-term health effects of titanium and the development of effective prevention strategies.
Conclusion
Testing for titanium toxicity is a complex and evolving field. While existing methods can provide valuable information about titanium exposure, limitations remain in terms of accuracy, accessibility, and interpretation. Ongoing research is focused on developing improved diagnostic tools and a better understanding of the mechanisms of titanium toxicity.
The development of standardized testing protocols and clinical guidelines is crucial for ensuring that individuals at risk of titanium toxicity receive appropriate medical care. As the use of titanium continues to expand, it is essential to prioritize research and address the potential health risks associated with this widely used material.
Ultimately, a collaborative effort involving researchers, clinicians, and regulatory agencies is needed to advance our understanding of titanium toxicity and protect public health.






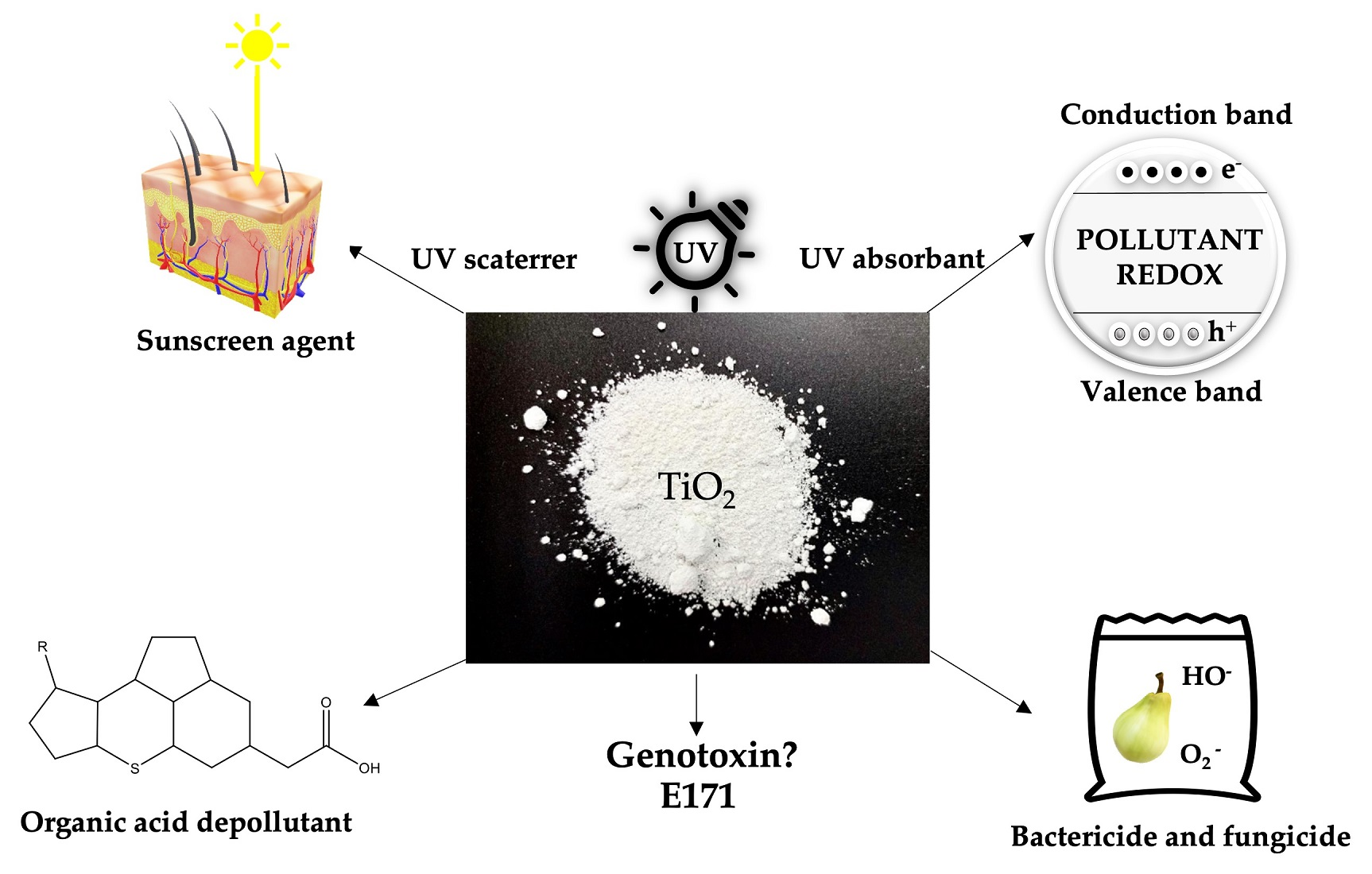




![How To Test For Titanium Toxicity [PDF] Titanium toxicity-A review | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/a26b3ca0b318cfdb4afe92578a617a43234c411f/2-Figure1-1.png)





