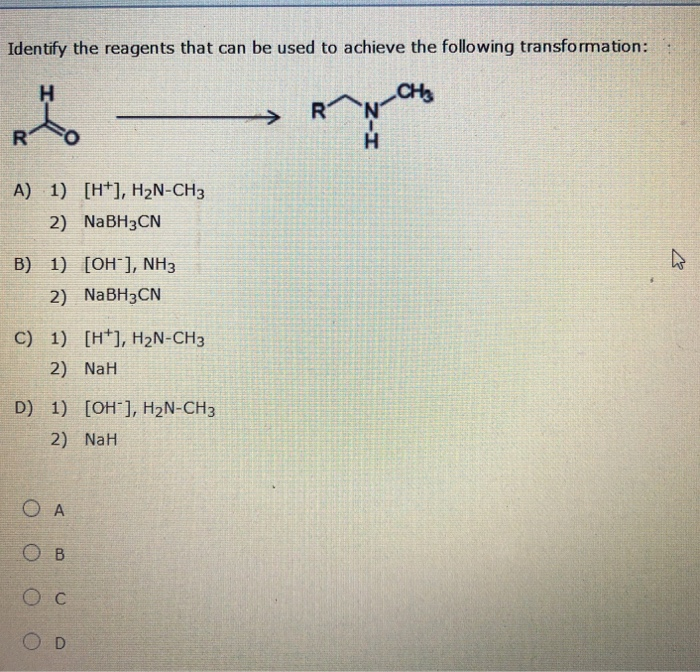
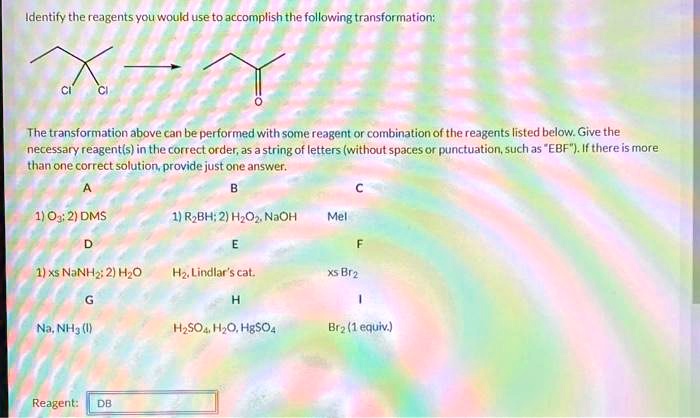
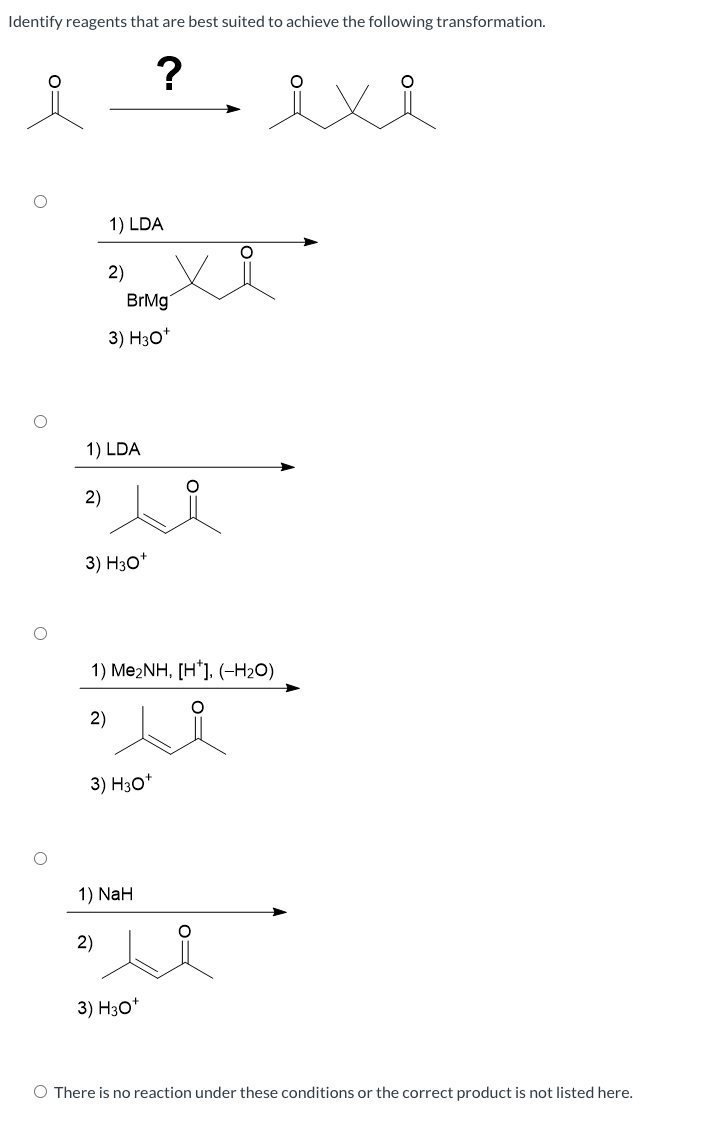
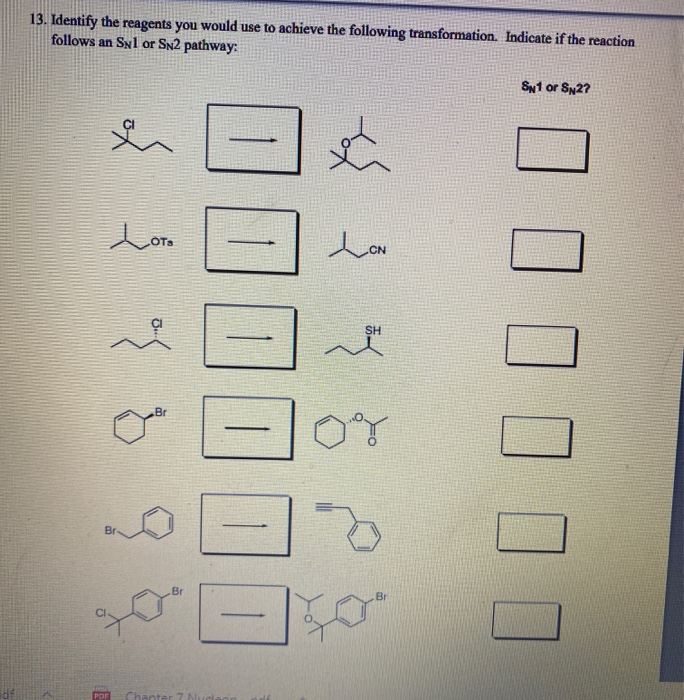
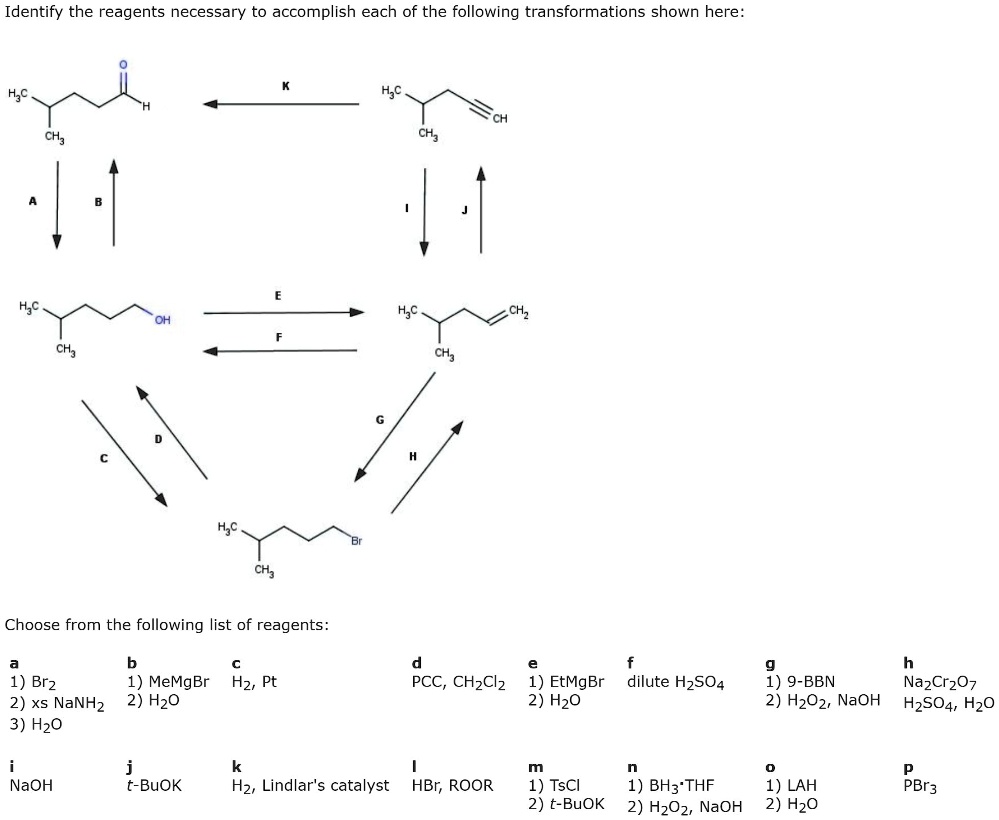
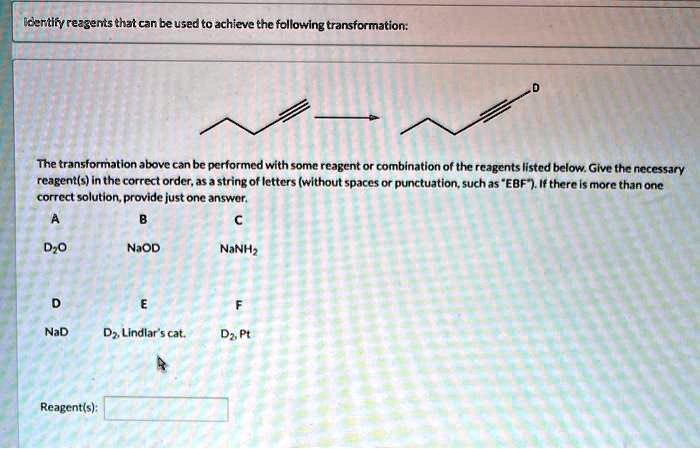
Identify The Best Reagents To Achieve The Following Transformation

Imagine you're a chef, not in a bustling restaurant kitchen, but in a laboratory. Your recipe isn't for a delicious meal, but for a complex molecule with potential life-changing applications. The ingredients aren't spices and vegetables, but carefully selected chemical reagents. The challenge? To transform one molecule into another, efficiently and selectively, using the best possible tools.
The heart of organic chemistry lies in the art and science of transforming molecules. Achieving a specific transformation requires choosing the right reagents, a process that demands a deep understanding of chemical reactivity and reaction mechanisms. The optimal reagents ensure a high yield of the desired product, minimize unwanted side reactions, and can significantly impact the efficiency and cost-effectiveness of chemical synthesis.
The Foundation: Understanding Organic Transformations
Organic transformations are the cornerstone of modern chemistry. They're essential for creating new materials, developing pharmaceuticals, and advancing various technological fields. The ability to selectively manipulate molecules, breaking and forming bonds at specific locations, opens doors to synthesize complex structures from simpler building blocks.
The selection of appropriate reagents for a transformation is a critical decision. It hinges on understanding the electronic properties of the starting material, the desired changes in the molecular structure, and the reaction conditions that will favor the desired pathway.
A Glimpse into Reagent Selection
Consider a seemingly simple task: the reduction of a ketone to a secondary alcohol. Several reagents can accomplish this, but each has its strengths and weaknesses. Sodium borohydride (NaBH4) is a common choice, known for its mildness and selectivity. It reduces ketones and aldehydes effectively without affecting other functional groups like esters or amides.
Lithium aluminum hydride (LiAlH4), on the other hand, is a more powerful reducing agent. While capable of reducing a wider range of functional groups, it requires more careful handling and may lead to undesired side reactions if not used judiciously.
Key Considerations for Reagent Selection
Several factors influence the choice of reagents for a specific organic transformation. These range from the reactivity of the functional groups involved to the overall atom economy and environmental impact of the reaction.
Selectivity is paramount. A good reagent will react preferentially with the desired functional group, leaving other parts of the molecule untouched. This prevents unwanted side products and simplifies the purification process.
Yield is another critical factor. The higher the yield of the desired product, the more efficient the transformation. Reagents that promote high yields minimize waste and conserve valuable starting materials.
Reaction conditions play a vital role. Some reagents require specific temperatures, solvents, or catalysts to function effectively. Understanding these requirements is essential for optimizing the reaction and achieving the desired outcome.
Cost and availability also influence reagent selection. Researchers often consider the expense of the reagents and their accessibility when designing a synthetic route. Sustainable alternatives are increasingly favored to minimize environmental impact and promote green chemistry principles.
Examples in Action
Let's examine a few common organic transformations and explore the reagents typically employed.
Oxidation of Alcohols
The oxidation of alcohols to aldehydes or ketones is a fundamental transformation in organic chemistry. Depending on the desired outcome, different oxidizing agents are used.
For instance, Pyridinium chlorochromate (PCC) is commonly used to oxidize primary alcohols to aldehydes. It selectively oxidizes the alcohol without further oxidizing the aldehyde to a carboxylic acid.
Alternatively, strong oxidizing agents like potassium permanganate (KMnO4) or chromic acid (H2CrO4) can oxidize primary alcohols directly to carboxylic acids. These reagents are more powerful and less selective than PCC.
Esterification
The formation of an ester from a carboxylic acid and an alcohol is known as esterification. This reaction can be catalyzed by acids, such as sulfuric acid (H2SO4) or hydrochloric acid (HCl).
Alternatively, the use of coupling reagents like dicyclohexylcarbodiimide (DCC) or 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) can facilitate ester formation under milder conditions. These reagents activate the carboxylic acid, making it more susceptible to nucleophilic attack by the alcohol.
Grignard Reactions
Grignard reactions are powerful tools for forming carbon-carbon bonds. These reactions involve the addition of a Grignard reagent (RMgX) to a carbonyl compound, such as an aldehyde or ketone.
The Grignard reagent acts as a nucleophile, attacking the electrophilic carbonyl carbon. This leads to the formation of a new carbon-carbon bond and the creation of a more complex molecule. Grignard reagents are highly reactive and require anhydrous conditions to prevent decomposition.
Beyond Traditional Reagents: Exploring New Frontiers
The field of reagent development is constantly evolving, with researchers seeking new and improved ways to carry out organic transformations. This includes the development of more selective, efficient, and environmentally friendly reagents.
Organocatalysis is an emerging area that utilizes organic molecules as catalysts. These catalysts can promote a wide range of reactions with high selectivity and efficiency, often under mild conditions. Organocatalysis offers a sustainable alternative to traditional metal-based catalysts.
Biocatalysis, the use of enzymes as catalysts, is another promising approach. Enzymes are highly selective and operate under mild conditions, making them ideal for synthesizing complex molecules with high stereochemical control. Biocatalysis is particularly useful in the pharmaceutical industry for producing chiral drug candidates.
The future of reagent development lies in innovation and a commitment to sustainability. By exploring new catalytic methods and designing environmentally friendly reagents, chemists can pave the way for more efficient and sustainable chemical processes.
The Path Forward: Mastering Reagent Selection
Choosing the best reagents for a given organic transformation requires a comprehensive understanding of chemical principles and a keen awareness of the available options. By carefully considering the factors discussed, chemists can optimize their reactions and achieve the desired outcomes.
Mastering reagent selection is a continuous learning process. Staying abreast of new developments in the field and gaining experience with different reagents are essential for success. The ability to choose the right reagents is a hallmark of a skilled organic chemist, enabling the creation of new molecules with potential applications in medicine, materials science, and beyond.
The selection of reagents isn't just a technical skill; it's an art form. It's about understanding the nuances of molecular interactions, predicting reaction pathways, and creatively solving chemical challenges. Ultimately, the best reagents are those that empower chemists to transform their visions into reality, one molecule at a time.