Incontinence Devices Medical Devices Pipeline Product Analysis

The global incontinence devices market is poised for explosive growth, driven by an aging population and increasing awareness of treatment options. Innovations in non-invasive and minimally invasive devices are reshaping the landscape, promising relief for millions suffering from bladder and bowel control issues.
This analysis dissects the current state of the incontinence devices pipeline, highlighting key players, emerging technologies, and potential market disruptors. The report focuses on confirmed product developments and market trends, providing a clear picture of the future of incontinence care.
Market Overview: A Growing Need
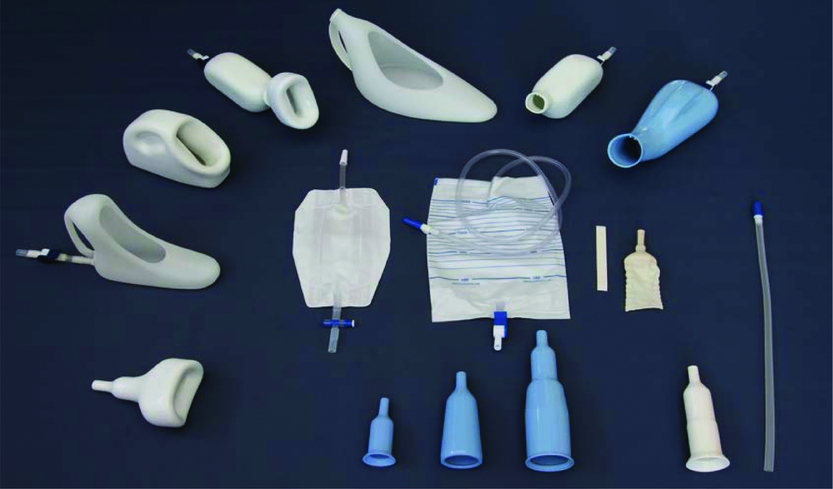
The incontinence devices market is segmented by product type, including urinary incontinence devices (e.g., artificial urinary sphincters, slings, and electrical stimulation devices) and fecal incontinence devices (e.g., bowel control systems and sacral nerve stimulation devices). Urinary incontinence devices currently dominate the market, but advancements in fecal incontinence solutions are expected to fuel significant growth.
According to a recent report by Global Market Insights, the incontinence devices market is projected to reach $4.8 billion by 2028, growing at a CAGR of over 6% from 2022. This growth is attributed to factors such as the rising prevalence of obesity, diabetes, and neurological disorders, all of which contribute to incontinence.
North America currently holds the largest market share, followed by Europe. However, the Asia Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing healthcare expenditure and growing awareness.
Key Players and Product Pipeline
Several major players dominate the incontinence devices market, including Boston Scientific, Medtronic, and Coloplast. These companies have established strong market positions through their extensive product portfolios and global distribution networks.
Boston Scientific is a leader in urinary incontinence solutions, with products like the AMS 800 Artificial Urinary Sphincter and the Solyx Single-Incision Sling System. The company is also investing in research and development of next-generation devices, including neuromodulation therapies.
Medtronic is a key player in fecal incontinence treatment, offering the InterStim System, a sacral nerve stimulation device that helps restore bowel control. Medtronic is actively pursuing clinical trials to expand the indications for InterStim and explore its potential in treating other pelvic floor disorders.
Emerging Technologies and Innovations
The incontinence devices pipeline is characterized by a growing focus on non-invasive and minimally invasive solutions. Researchers are exploring novel technologies such as transcutaneous electrical nerve stimulation (TENS) and magnetic stimulation to treat urinary incontinence.
Another promising area of innovation is the development of smart incontinence devices that can monitor bladder activity and provide personalized treatment recommendations. These devices utilize sensors and algorithms to track urinary frequency, volume, and leakage, allowing for more targeted and effective management of incontinence.
UroMedical Corporation is developing the MGuard, a minimally invasive urethral bulking agent for stress urinary incontinence. The company recently announced positive results from a clinical trial demonstrating the safety and efficacy of MGuard in women with SUI.
Regulatory Landscape and Market Access
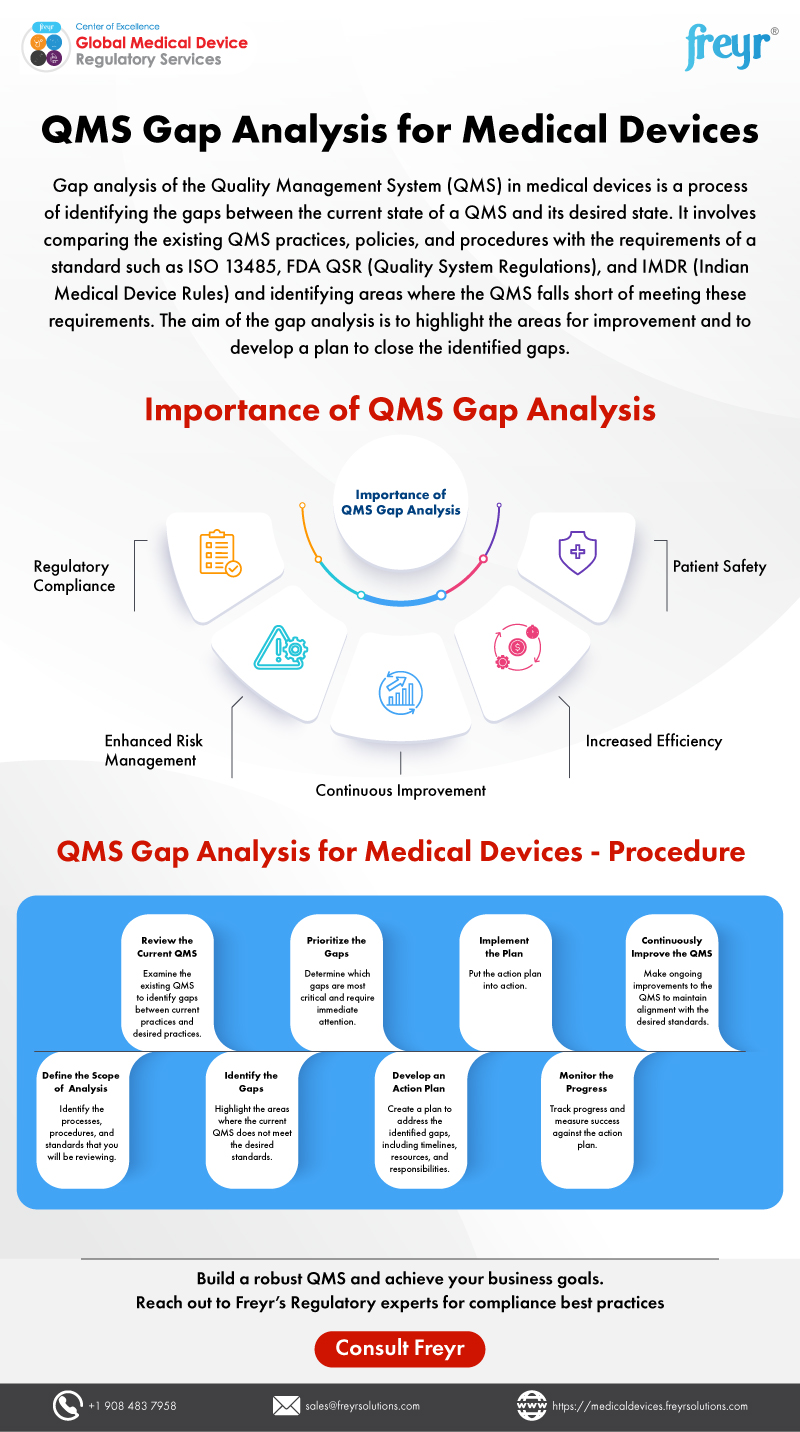
Incontinence devices are subject to stringent regulatory requirements in most countries. In the United States, the Food and Drug Administration (FDA) regulates these devices through the Premarket Approval (PMA) or 510(k) clearance pathways, depending on the risk profile of the device.
Obtaining regulatory approval can be a lengthy and expensive process, requiring extensive clinical trial data and rigorous safety testing. Companies seeking to enter the incontinence devices market must navigate these regulatory hurdles to gain market access.
Reimbursement policies also play a crucial role in determining the commercial success of incontinence devices. In many countries, reimbursement for these devices is limited, which can restrict patient access and limit market growth. Advocacy efforts are underway to improve reimbursement policies and expand coverage for incontinence care.
Challenges and Opportunities
Despite the promising growth prospects, the incontinence devices market faces several challenges. These include the stigma associated with incontinence, limited awareness of treatment options, and high device costs.
However, there are also significant opportunities for growth. Increasing awareness of incontinence, coupled with the development of innovative and affordable devices, can drive market expansion. Telehealth and remote monitoring technologies can also improve access to care and enhance patient outcomes.
The Incontinence Foundation is actively working to raise awareness and provide support to individuals affected by incontinence. Their efforts are crucial in breaking down the stigma associated with this condition and promoting access to effective treatment.
Conclusion: The Road Ahead
The incontinence devices market is undergoing a period of rapid innovation and growth. The pipeline is filled with promising new technologies that have the potential to significantly improve the lives of millions of people suffering from bladder and bowel control issues.
Ongoing clinical trials and regulatory approvals will shape the future of the market. Investors and industry stakeholders are closely monitoring these developments, seeking to capitalize on the growing demand for effective incontinence solutions. Further updates will be provided as new data emerges and products advance through the regulatory process.