Is An Atom Smaller Than A Cell
Urgent reports confirm fundamental science knowledge: an atom is definitively smaller than a cell. This isn't new discovery, but a crucial reaffirmation of basic biological and physical principles, essential for understanding life and matter.
This article clarifies the vast scale difference between atoms and cells, preventing potential misconceptions. Understanding this disparity is paramount for grasping complex scientific processes across various fields.
The Immense Scale Difference
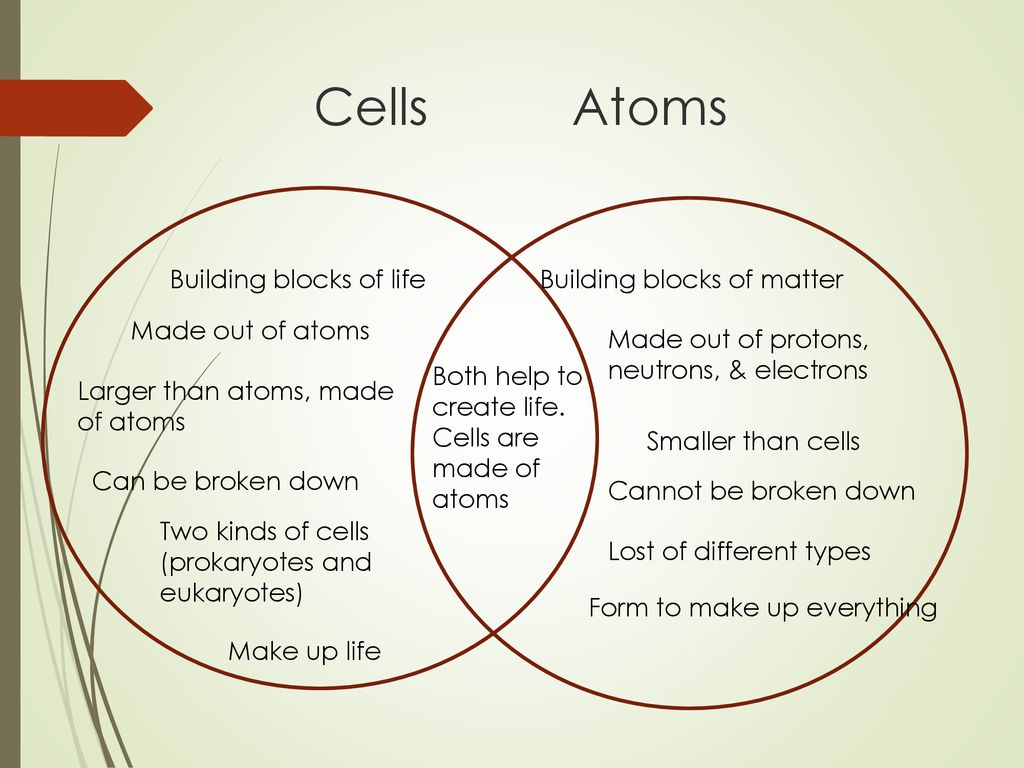
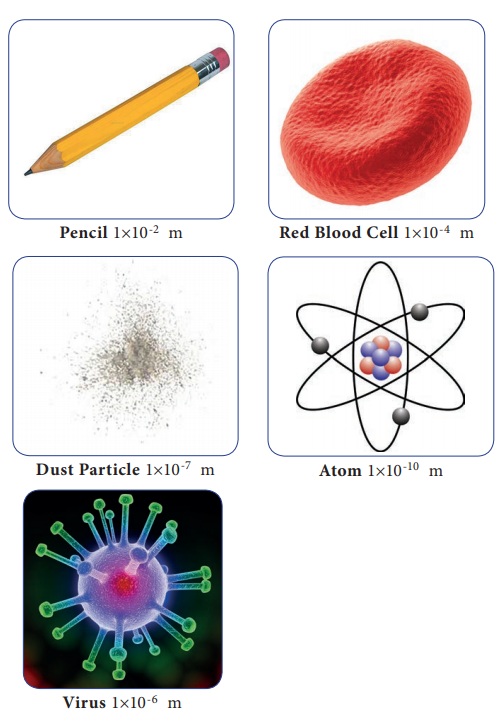
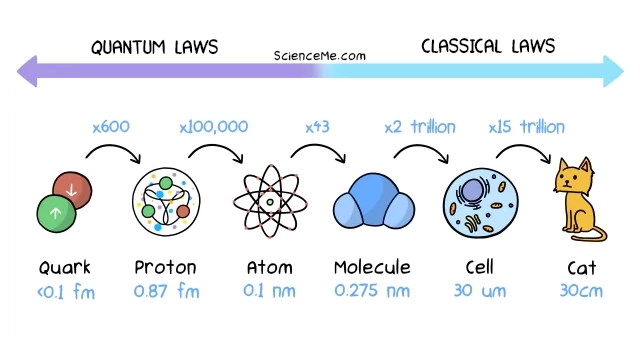
Atoms, the fundamental building blocks of matter, are measured in angstroms (Å), where 1 Å = 0.1 nanometers (nm). Typical atomic radii range from 0.3 to 3 Å. To put it simply, atoms are extremely small.
Cells, the basic units of life, are substantially larger. Their sizes are measured in micrometers (µm), where 1 µm = 1,000 nm. This means cells are thousands of times larger than atoms.
Animal cells typically range from 10 to 30 µm in diameter. Plant cells are generally larger, ranging from 10 to 100 µm.
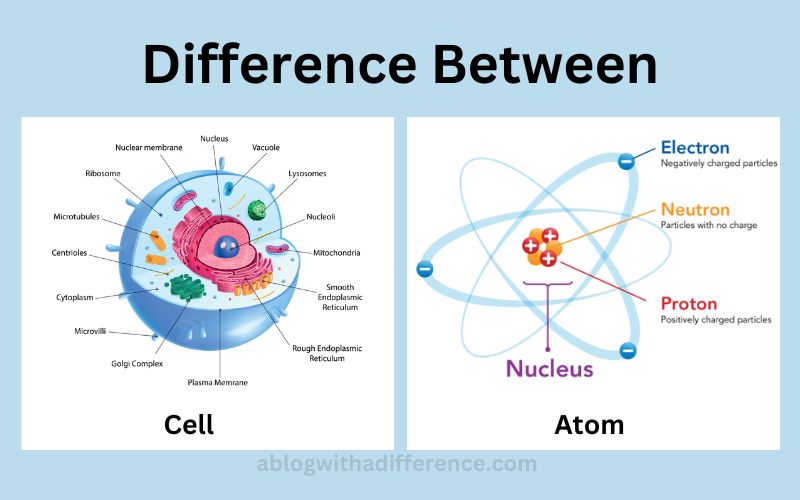
Atomic Structure: The Foundation
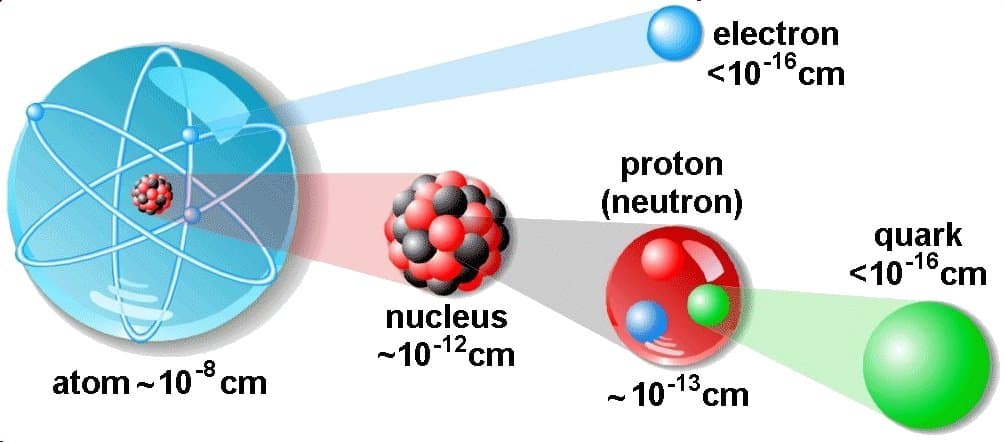
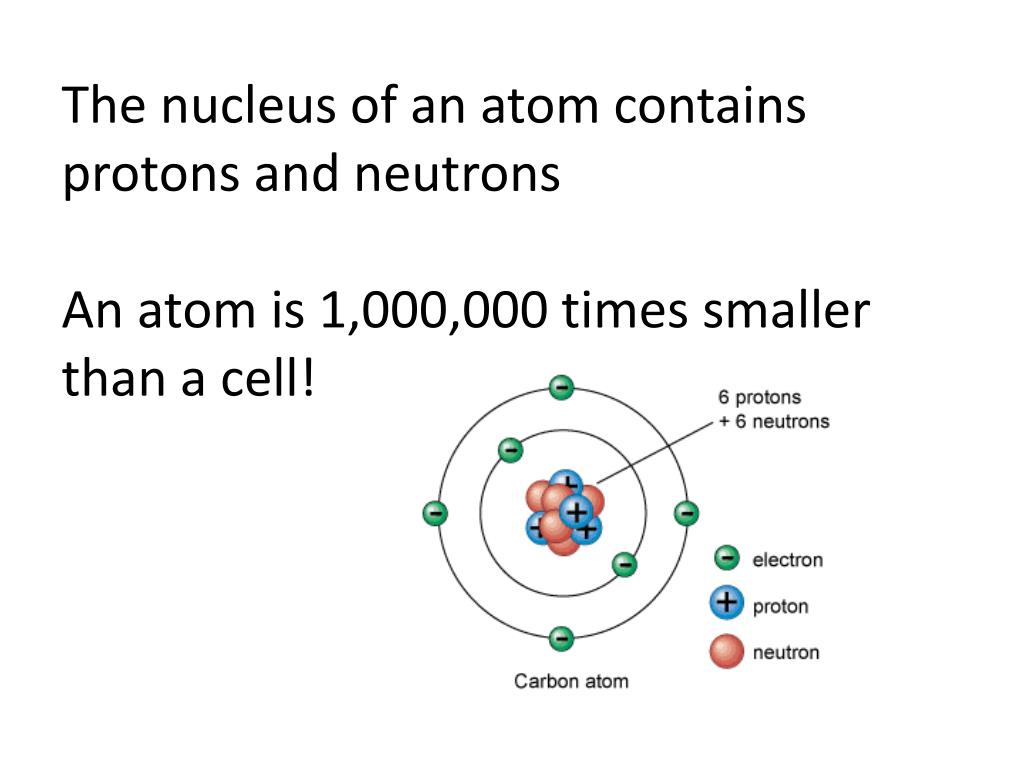
An atom consists of a nucleus containing protons and neutrons, surrounded by electrons. These subatomic particles dictate the atom's properties.
The number of protons determines the element (e.g., hydrogen, oxygen, carbon). The arrangement and behavior of electrons dictate how atoms interact and form molecules.
Cellular Structure: Complexity at Scale
Cells are incredibly complex structures containing various organelles, such as the nucleus, mitochondria, and endoplasmic reticulum.
These organelles perform specific functions essential for cell survival. The nucleus, for instance, houses the cell's DNA, the blueprint for all cellular activities.
Mitochondria are responsible for energy production, while the endoplasmic reticulum plays a role in protein synthesis and transport.
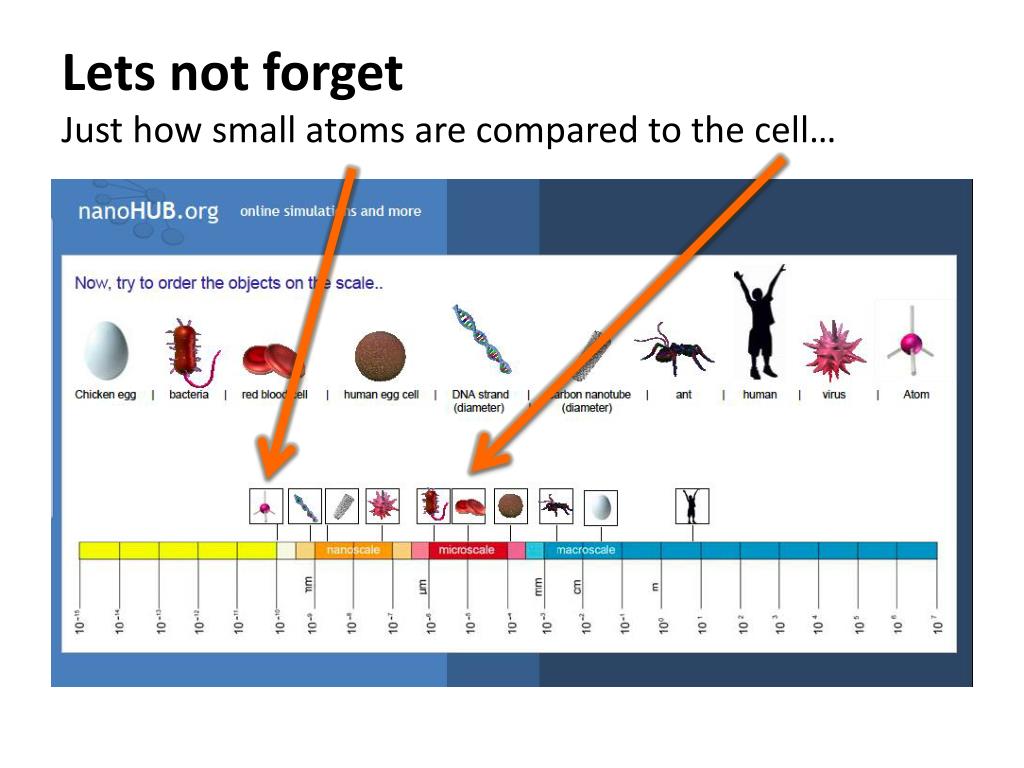
Quantifying the Difference: A Numerical Perspective
Consider a typical animal cell with a diameter of 20 µm (20,000 nm). Compare this to a carbon atom with a radius of approximately 0.07 nm.
This highlights the massive scale difference. It would take approximately 285,714 carbon atoms lined up to span the diameter of a single animal cell.
Therefore, a cell is orders of magnitude larger than an atom. This is not a subtle difference, but a fundamental characteristic of biological and physical organization.
Why This Matters
Understanding the size difference is crucial for numerous scientific disciplines. For example, drug delivery systems target specific cells, necessitating the design of nanoparticles much smaller than the cells themselves.
In materials science, understanding atomic arrangements dictates the properties of materials at the macroscopic level. The behavior of individual atoms influence the characteristics of materials.
In biology, comprehending molecular interactions at the atomic level is essential for understanding cellular processes. Interactions between molecules within the cell drive its function.
Addressing Misconceptions
Misconceptions about the relative sizes of atoms and cells can lead to confusion. It is important to constantly reiterate and reinforce these fundamental scientific concepts.
Educational resources should emphasize clear visual representations of scale. This helps solidify understanding and prevents potential errors.
Simple analogies can also be helpful. For instance, comparing an atom to a grain of sand and a cell to a football field can provide a sense of the scale difference.
Ongoing Developments and Future Directions
Research continues to push the boundaries of nanotechnology. Scientists are developing tools and techniques to manipulate matter at the atomic level.
Advances in microscopy allow us to visualize cellular structures with unprecedented detail. This leads to a better understanding of how cells function and how diseases develop.
Continued efforts in science education are crucial for promoting scientific literacy. Ensuring a strong foundation in basic scientific principles is crucial for the next generation.
Key Takeaway: An atom is significantly smaller than a cell. This foundational knowledge is critical for understanding a wide range of scientific phenomena. It is essential to continually reinforce this concept through education and research.
Efforts will continue to emphasize this scale in educational materials, ensuring basic concepts are clearly conveyed. Further research will build on this foundation, driving innovations in medicine, materials science, and beyond.