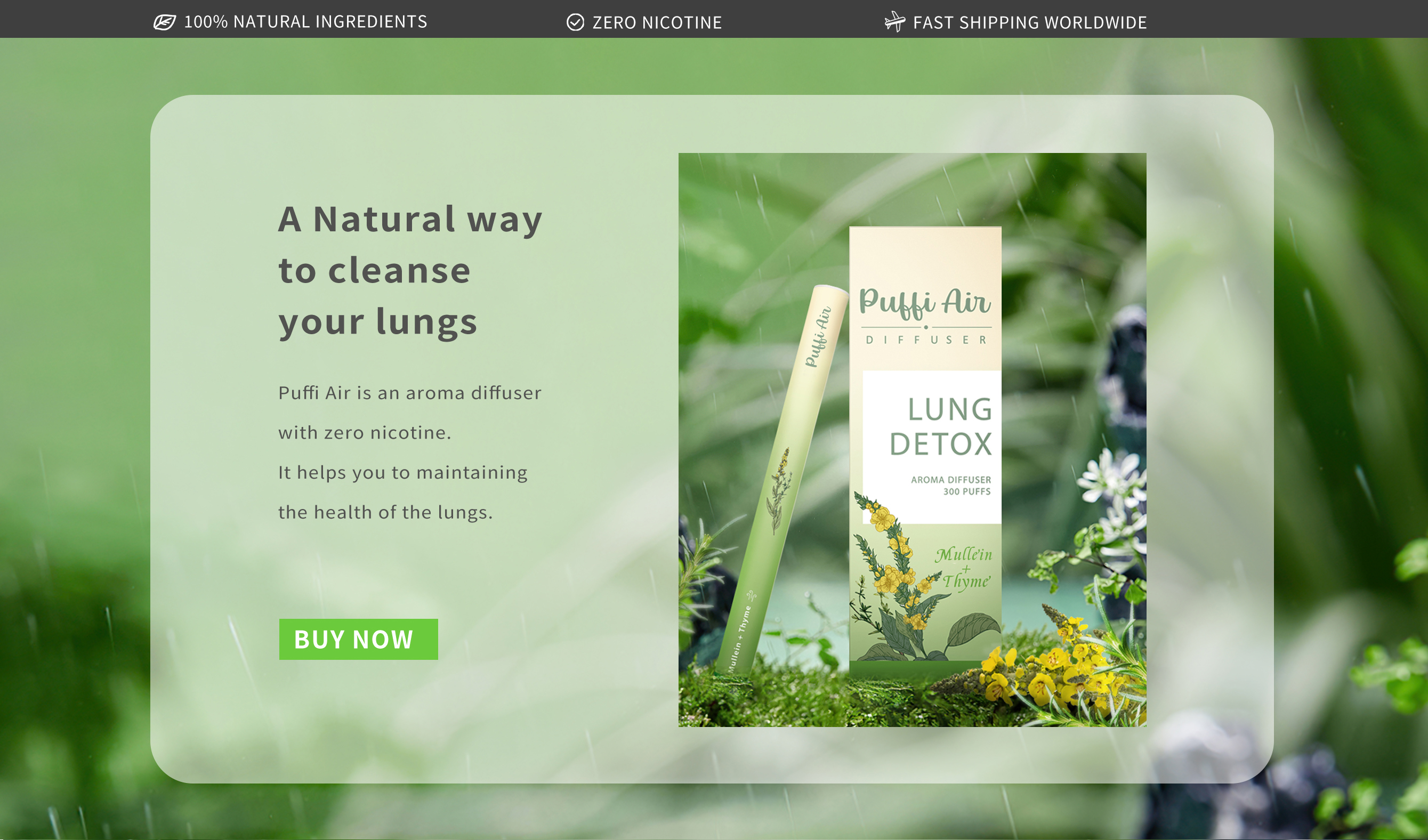
Is Puffi Air Lung Detox Safe

Concerns are mounting over the safety of Puffi Air Lung Detox, a popular over-the-counter respiratory supplement, as reports of adverse reactions surge. Health officials are urgently investigating the product's ingredients and potential health risks following a wave of hospitalizations.
The controversy surrounding Puffi Air Lung Detox highlights the potential dangers of unregulated health supplements. This article breaks down the known facts about the product's safety, the reported adverse effects, and the ongoing investigations.
Who is Affected?
Reports indicate that individuals with pre-existing respiratory conditions, such as asthma and COPD, are particularly vulnerable. However, healthy individuals have also reported experiencing negative side effects after using Puffi Air Lung Detox.
Children and elderly individuals are considered to be at higher risk. The exact number of affected individuals is currently under investigation, but local hospitals confirm a significant increase in respiratory-related admissions potentially linked to the supplement.
What is Puffi Air Lung Detox?
Puffi Air Lung Detox is marketed as a natural supplement designed to cleanse the lungs and improve respiratory function. It is widely available online and in various retail stores, often promoted for individuals exposed to pollutants or those seeking to quit smoking.
The product's label lists a combination of herbal extracts and vitamins. However, specific ingredients and their concentrations are now under scrutiny by regulatory agencies.
Where are the Incidents Occurring?
Initial reports of adverse reactions originated in several states, including California, Texas, and Florida. The Centers for Disease Control and Prevention (CDC) is now tracking cases nationwide to establish a clear geographic pattern.
Several online forums and social media groups have emerged, with users sharing their negative experiences and raising concerns about the product's safety.
When Did the Problems Start?
The surge in reported adverse effects began approximately three months ago, coinciding with an aggressive marketing campaign for Puffi Air Lung Detox. Prior to this period, reports were minimal and scattered.
Health professionals noticed an uptick in patients presenting with similar symptoms shortly after the marketing push.
How are People Affected?
Reported adverse effects range from mild to severe, including shortness of breath, chest pain, nausea, and dizziness. In more severe cases, individuals have experienced acute respiratory failure requiring hospitalization and intensive care.
Some patients have reported allergic reactions, including skin rashes and swelling. The severity of the symptoms varies depending on the individual's health status and the dosage of the supplement consumed.
Authorities are investigating whether the reported symptoms are directly caused by Puffi Air Lung Detox ingredients or are related to contamination during manufacturing.
The Investigation
The Food and Drug Administration (FDA) has launched an official investigation into Puffi Air Lung Detox. This includes testing samples of the product for contaminants and verifying the accuracy of the ingredient list.
"We are taking these reports very seriously," stated Dr. Emily Carter, a lead investigator at the FDA. "Our priority is to determine the safety of this product and protect the public from potential harm."
The manufacturer of Puffi Air Lung Detox, NutriLife Supplements, has issued a statement claiming that their product is safe and effective. However, they have agreed to cooperate with the FDA's investigation.
Expert Opinions
Pulmonologists and toxicologists are urging consumers to immediately discontinue use of Puffi Air Lung Detox. They emphasize that there is no scientific evidence to support the product's claims of lung detoxification.
"There is no such thing as a 'lung detox' in the way it's being marketed," explains Dr. Michael Davis, a leading pulmonologist. "The lungs are self-cleaning organs. These supplements can potentially cause more harm than good."
What Should You Do?
If you are currently using Puffi Air Lung Detox, discontinue use immediately and consult with your healthcare provider. Report any adverse reactions to the FDA through their MedWatch program.
Be wary of health supplements that make unsubstantiated claims. Always consult with a qualified healthcare professional before taking any new supplement, especially if you have underlying health conditions.
Next Steps
The FDA investigation is ongoing, and further updates will be released as more information becomes available. Health officials are urging consumers to remain vigilant and informed about the potential risks associated with health supplements.
NutriLife Supplements may face legal action and product recalls depending on the outcome of the FDA's investigation. The long-term health consequences for individuals who have experienced adverse reactions are still being evaluated.